Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG1-nd |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Pritumumab Biosimilar - Anti-VIM mAb - Research Grade |
|---|---|
| Source | CAS 499212-74-7 |
| Species | Homo sapiens |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Pritumumab,CLN G11,VIM ,anti-VIM |
| Reference | PX-TA1193 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG1-nd |
| Clonality | Monoclonal Antibody |
Pritumumab Biosimilar: A Promising Antibody for Targeting VIM in
Pritumumab biosimilar, also known as anti-VIM monoclonal antibody (mAb), is a novel therapeutic agent that has shown great potential in targeting VIM (vimentin) in cancer treatment. VIM is a type III intermediate filament protein that plays a crucial role in maintaining cellular integrity and promoting cell migration. However, overexpression of VIM has been linked to cancer progression and metastasis in various types of tumors, making it an attractive therapeutic target. In this article, we will discuss the structure, activity, and potential applications of Pritumumab biosimilar as an anti-VIM mAb in cancer research.
Pritumumab biosimilar is a recombinant humanized IgG1 monoclonal antibody that specifically targets the N-terminal domain of VIM. It is produced through genetic engineering techniques, where the variable region of the antibody is derived from a mouse anti-VIM mAb and fused with human constant regions. This results in a chimeric antibody that retains the specificity and affinity of the mouse mAb while minimizing the risk of immunogenicity in humans.
The antibody has a molecular weight of approximately 150 kDa and consists of two identical heavy chains and two identical light chains. The heavy chains contain four constant domains (CH1, CH2, CH3, and CH4) and one variable domain (VH), while the light chains consist of one constant domain (CL) and one variable domain (VL). The variable regions of both chains are responsible for binding to the N-terminal domain of VIM, while the constant regions determine the antibody’s effector functions.
cancer effects by binding to VIM on the surface of cancer cells and triggering a series of downstream signaling pathways. This leads to inhibition of VIM-mediated cell migration, invasion, and metastasis. Additionally, the antibody can also induce antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) by recruiting immune cells and complement proteins to eliminate cancer cells.
Several preclinical studies have demonstrated the efficacy of Pritumumab biosimilar in inhibiting tumor growth and metastasis in various cancer models, including breast, lung, and colon cancer. In a recent phase I clinical trial, Pritumumab biosimilar showed promising results in patients with advanced solid tumors, with manageable safety profile and no dose-limiting toxicities reported.
Potential Applications in
The ability of Pritumumab biosimilar to specifically target VIM makes it a potential candidate for cancer therapy. VIM is overexpressed in many types of cancer, including breast, lung, colon, and pancreatic cancer, and has been associated with poor prognosis and drug resistance. By targeting VIM, Pritumumab biosimilar could potentially inhibit cancer cell migration and invasion, thereby preventing metastasis and improving patient outcomes.
Moreover, the antibody’s dual mechanism of action, i.e., inhibiting VIM-mediated cell migration and inducing immune-mediated cytotoxicity, makes it a promising candidate for combination therapy with other anti- cancer agents. In fact, a phase II clinical trial is currently underway to evaluate the efficacy and safety of Pritumumab biosimilar in combination with chemotherapy in patients with metastatic breast cancer.
In conclusion, Pritumumab biosimilar is a novel antibody that specifically targets VIM, a key player in cancer progression and metastasis. Its unique structure and mechanism of action make it a promising candidate for cancer therapy, and ongoing clinical trials are expected to further validate its efficacy and safety. With its potential to improve patient outcomes and overcome drug resistance, Pritumumab biosimilar holds great promise in the field of cancer research and treatment.

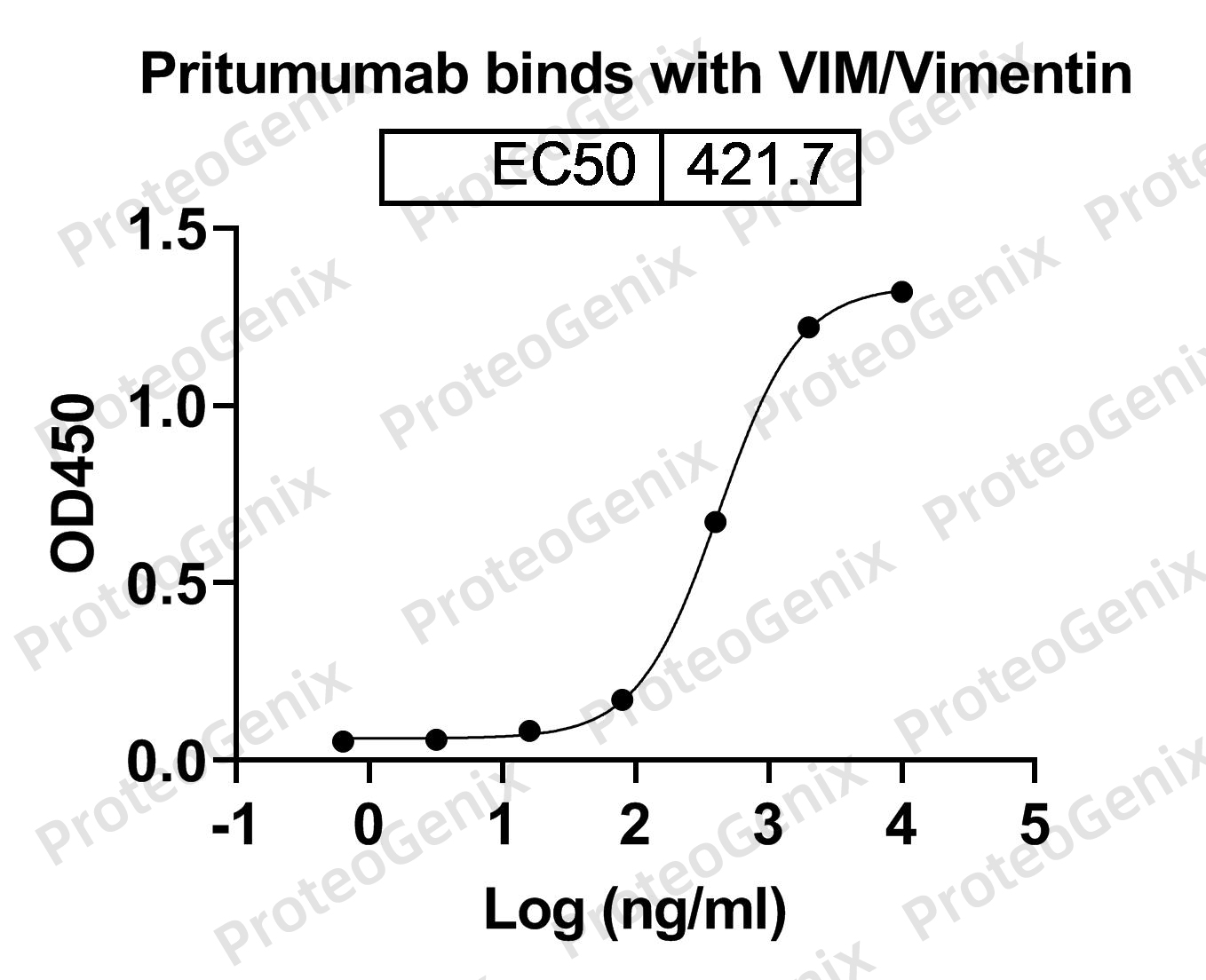
Immobilized VIM / Vimentin, N-His, recombinant protein (cat. No.PX-P5979) at 0.5µg/mL (100µL/well) can bind to Pritumumab Biosimilar - Anti-VIM mAb (cat. No.PX-TA1193) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Send us a message from the form below
Reviews
There are no reviews yet.