 CAR-T cell therapy
CAR-T cell therapy
From the first to the fifth generation of CAR-T cells
Prompted by encouraging clinical and preclinical results, the structure of CAR-T cells has evolved rapidly to minimize off-target toxicity and increase antigen-specific activation. Most changes have occurred in the intracellular domain due to its importance for CAR-T cell activation and proliferation. Currently, CAR-T cells can be classified into four different generations, with next or fifth-generation CARs currently under active development. In this article, we offer an overview of the most important feature of each generation of CAR-T cell therapies and explore the most promising research trends.
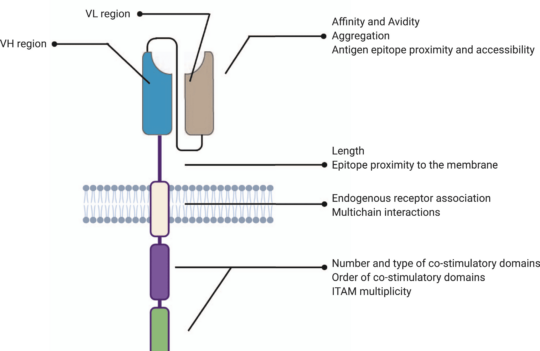
Chimeric antigen receptor (CAR) T cell therapy was first proposed in the late 80s. Their general structure consists of an ectodomain containing antigen-binding and spacer domains, a transmembrane domain serving as the anchor to the T cell membrane, and an intracellular or endodomain responsible for initiating the signaling cascade. Although their fundamental modular structure has remained similar since their inception, CAR-T cells can be classified into five generations according to the organization of their intracellular signaling domain.
Why have structural changes of CARs focused mostly on this intracellular region? Because CARs are designed based on the principles of TCR (T cell receptors) and costimulatory signaling.
In these structures, the intracellular domains work as the functional endpoints by triggering differentiation, cytotoxic response, cytokine production, and by recruiting other immune cells that will enhance the process of tumor elimination. These mechanisms allow a non-MHC restricted targeting of tumors. For this reason, most strategies aiming to enhance CAR-T cell clinical efficacy have focused on amplifying and sustaining these signaling pathways.
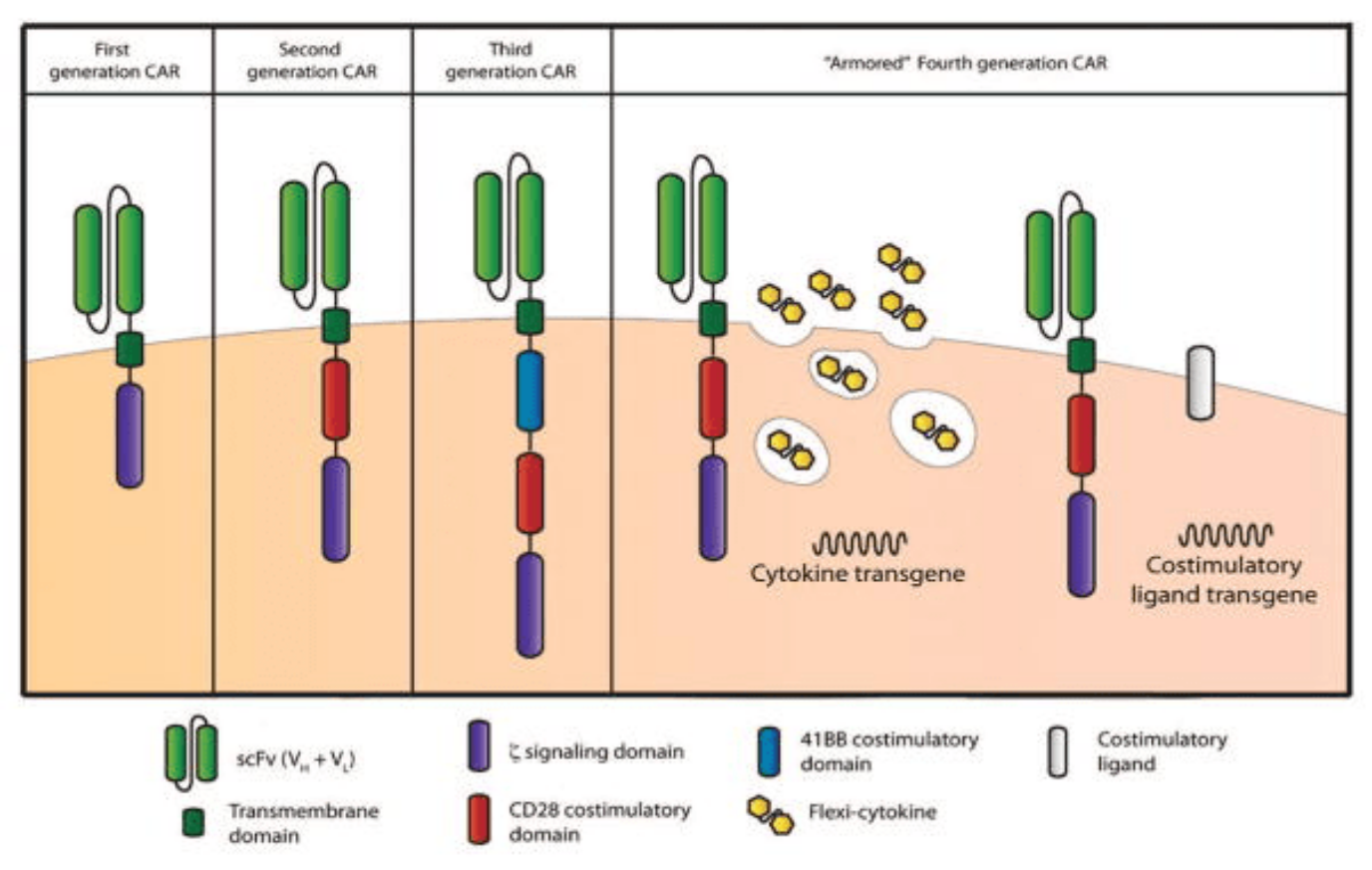
First-generation CAR-T cells
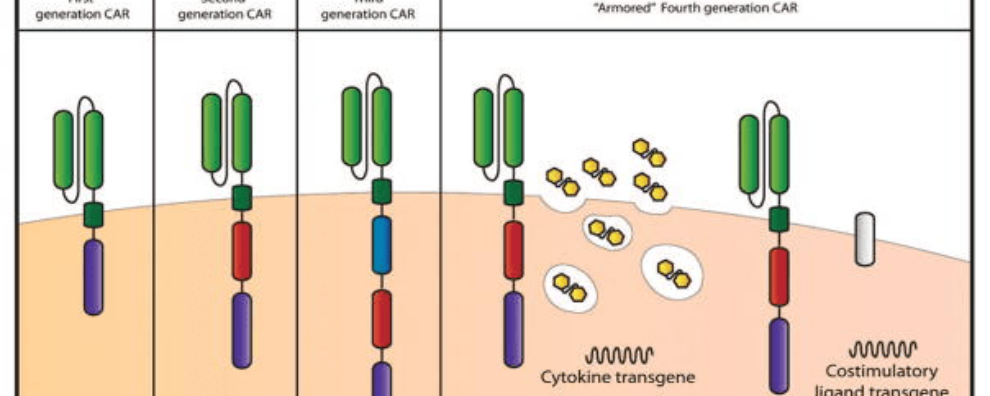
First-generation CARs contained a single CD3 ζ- chain or FcεRIγ intracellular domain devoid of additional costimulatory domains. These complexes were very similar to endogenous TCR; however, they suffered from one major drawback – the inability to produce sufficient IL-2 (interleukin-2). Given their weak response, first-generation CARs had to be supplemented with exogenous IL-2 to ensure an efficient response. Moreover, studies revealed these modified cells still displayed low cell proliferation and short in vivo lifespan, which further prompted the development of costimulatory domains.
Second-generation CAR-T cells
Second-generation CARs attempted to solve the challenges caused by inadequate proliferation, low cytokine production, and the short lifespan of conventional CAR-T cells. They did this by leveraging the power of dual signaling known to drive strong T cell proliferation in natural systems.
This new generation of CARs contained additional cytoplasmic domains such as CD28, 4-1BB, or OX-40, capable of delivering a secondary signal upon encountering a tumor antigen. Clinical and preclinical studies revealed that the presence of the costimulatory signal was able to improve proliferation, cytotoxicity, and sustained response due to longer in vivo half-lives.
Studies also revealed that the composition of the costimulatory domain played a vital role in modulating these parameters. For instance, some reports revealed that 4-1BBζ-CAR-T cells might persist longer in circulation than CD28ζ-CAR-T. However, while the first may cause early exhaustion of CAR-T cells, the second was also reported to lead to the constitutive stimulation (activation in the absence of the antigen). For this reason, CAR design has evolved into better costimulatory constructs.
Third-generation CAR-T cells
Third-generation CARs have been made by combining multiple costimulatory signaling domains within the endodomain. Known examples of these constructs include CD3ζ-CD28-OX40 or CD3ζ-CD28-41BB. The latter was particularly promising since CD28 costimulatory domains are well-known for their ability to trigger rapid tumor elimination while 4-1BB endodomains promote the long-term persistence of CARs. Although these have been used to successfully treat certain types of cancer with good safety profiles, increased persistence, and proliferation, no enhanced efficacy was achieved in comparison to second-generation CAR-T cells.
Fourth-generation or TRUCK CAR-T cells
Given that the presence of multiple costimulatory domains failed to improve CAR-T cell efficacy, fourth-generation CARs were based on second-generation constructs. The difference between the two generations is that the latest is additionally modified with a constitutive or inducible expression cassette containing a transgenic protein such as a cytokine.
These are called T cell redirected for universal cytokine-mediated killing (TRUCK) CAR-T cells and they are designed to deliver the transgenic product to the targeted tumor site. This is usually achieved by engineering these cells to carry a nuclear factor of the activated T cell (NFAT)-responsive cassette (containing the transgenic cytokine such as IL-12). The expression of the transgene is thus induced when CD3ζ-containing CARs engage with their specific target. In practice, the engineering of TRUCK CAR-T cells requires the transfer of two transgenic cassettes – one for the CAR structure and another for the inducible cytokine.
In preclinical models, the presence of a cytokine transgene greatly enhanced the efficacy of CAR-T cell therapies in comparison to second-generation CARs. Moreover, the approach was also successful at avoiding systemic toxicity – one of the most common drawbacks of CAR-T cell therapy.
Next or fifth generation CAR-T cells
CAR-T cell therapies have greatly evolved during the past years in an attempt to enhance persistence, proliferation, safety, and efficacy. However, it remains challenging to minimize CAR-T cells’ off-target and off-tumor toxicity.
In this context, next-generation CAR-T cells are currently underway. The new generation, already named the fifth generation by experts, differs from the previous ones because they integrate an additional membrane receptor.
In TRUCKS or fourth-generation CARs, these modified T cells are activated by coming into contact with their target antigen, which leads to the induction of the secondary transgene, subsequent transcription, and secretion into the extracellular fluid. In this approach, the secreted signal not only stimulates CAR-T cells to remain active and form memory T cells but also reactivates the immune system to respond to restimulation.
CAR-T cells that use membrane receptors (fifth generation) act according to a different principle. Several approaches are currently being tested, the most promising relies on the addition of IL-2 receptors that allows JAK/STAT pathway activation in an antigen-dependent manner.
Other approaches have been tested but one of the most exciting developments of the field was the discovery of switch receptors. The successful incorporation of a drug-dependent OFF-switch leading to CAR depletion or an ON-switch leading to activation has recently been reported.
Based on these principles, lenalidomide-gated CARs were produced and tested. Although these cells proved to be slightly less efficacious in vitro, they were much more controllable than earlier generations of CARs resulting in a better safety profile and wider therapeutic window.
Concluding remarks
In the past years, the structure of CARs has evolved rapidly in an attempt to make these therapies safer. Although ectodomains and transmembrane domains have essentially remained the same since the inception of CARs, the endodomains have continued to evolve.
At the moment, five different generations of CAR-T cells have been identified, each employing different strategies to ensure the activation and proliferation of these modified T cells in response to a specific antigen. One of the most promising approaches has been the incorporation of a molecular switch into the CAR system, ensuring these therapeutics are either activated or degraded in response to chemical signals (i.e., drugs).
As researchers continue to pursue alternative strategies to improve this innovative technology, more breakthroughs are expected in the years to come.
- Brentjens, R. J. and Curran, K. J. Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematology Am Soc Hematol Educ Program. 2012; 2012: 143–151. doi: 10.1182/asheducation-2012.1.143
- Chmielewski, M. and Abken, H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015; 15(8):1145-1154. doi: 10.1517/14712598.2015.1046430
- Jan, M. et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci Transl Med. Sci Transl Med. 2021; 13(575): eabb6295. doi: 10.1126/scitranslmed.abb6295
- Subklewe, M. et al. Chimeric Antigen Receptor T Cells: A Race to Revolutionize Cancer Therapy. Transfus Med Hemother. 2019; 46(1): 15–24. doi: 10.1159/000496870
- Zhang, C. et al. Engineering CAR-T cells. Biomark Res. 2017; 5: 22. doi: 10.1186/s40364-017-0102-y