 Antibody production
Antibody production
The benefits of pairing monoclonal and polyclonal antibodies for custom immunoassay development
In a previous article, we highlighted the benefits and drawbacks of using monoclonal and polyclonal antibodies. In this article, we discuss how pairing the two types of antibodies can boost the efficiency of several immunoassays.
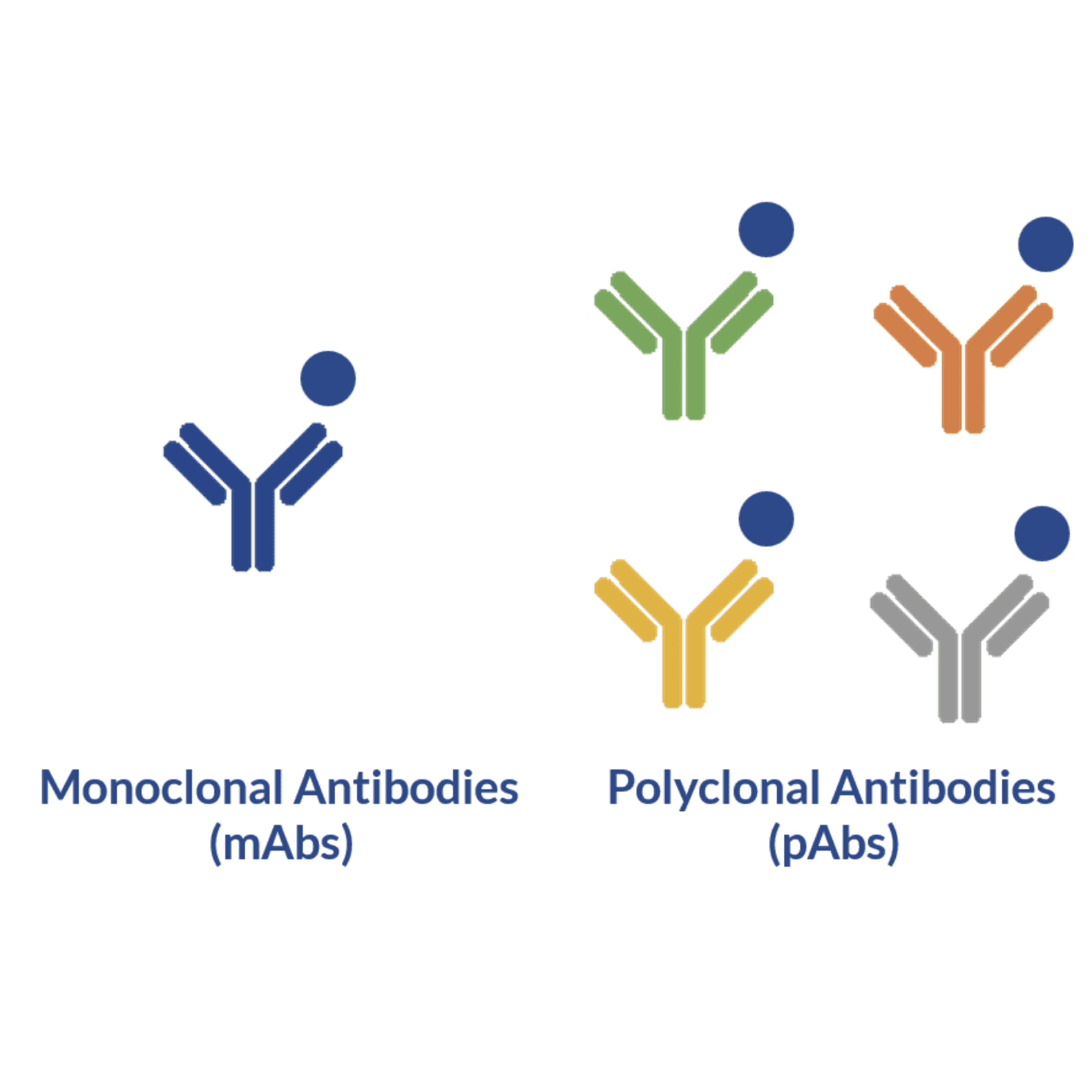
Immunoassays using monoclonal and polyclonal antibodies
Monoclonal antibodies have high affinity and specificity towards many biological molecules. This characteristic makes them highly valuable molecules for therapy and quantitative analytical assays. However, when the target antigen occurs in low abundance, the use of monoclonal antibodies alone may be insufficient to capture and detect them in clinical and environmental samples.
In these cases, it crucial to increase the sensitivity of the immunoassay without compromising its specificity. In research, this compromise is often achieved by pairing monoclonal and polyclonal antibodies.
Brief description of the main types of immunoassays
- Immunoprecipitation (IP) – it’s a small-scale affinity purification method that makes use of an antibody immobilized in a solid support (e.g. agarose resin or magnetic particles). It is commonly used to isolate or enrich biomolecules from cell or tissue lysates for downstream detection of target molecules.
- Chromatin immunoprecipitation (ChIP) – this assay is used to study protein-DNA interactions. It can be used to determine which proteins(s) are associated with different regions of the genome.
- Western Blot (WB) – it’s a widely used analytical method for detection and transferring of proteins in tissue homogenates or extracts.
- Immunohistochemistry (IHC) staining – this method relies on antibody-antigen complex to detect and diagnose the presence of specific markers in a given sample, such as cancer markers.
- Enzyme-linked immunosorbent assay (ELISA) – this highly versatile assay is used to detect and quantify specific target antigens across many different types of samples. Contrary to the other immunoassays, ELISA is more compatible with miniaturization and, for this reason, it is recurrently used for screening purposes during key steps of antibody generation and development processes.
Benefits of using polyclonal antibodies to increase sensitivity of different immunoassays
Performing immunoassays experiment is often a time-consuming task. For this reason, it is important to make the best decisions about assay design early on. And the decision to use monoclonal and polyclonal antibodies should be made right at the beginning of the project.
Although monoclonal antibodies remain the ideal molecules for countless applications, some specific immunoassays may actually benefit from the use polyclonal antibodies. These robust antibodies, often paired with monoclonal antibodies, are known to increase the sensitivity and robustness of these techniques.
This increased sensitivity results from the clonal diversity of these molecules. Due to their diversified nature, polyclonal immunoglobulins can target the entire antigen instead of just a small fraction or epitope of the target. This occurs because these molecules are designed to bind multiple sites (i.e. epitopes) of the antigen. Moreover, due to this broad binding range, several antibodies can bind to the same antigen resulting in signal amplification and more effective capture of the target antigen, which is highly desirable for IP and IHC techniques.
In techniques for screening and detection purposes, such as indirect ELISA, the use of antibody pairings with the polyclonal antibody as the secondary antibody is known to increase the sensitivity of the assay. This happens because these polyclonal molecules can be designed to target monoclonal antibodies. Thus, when the monoclonal antibody recognizes and binds the antigen, multiple polyclonal antibodies will bind to the same antigen-antibody complex. In these cases, the signal will be amplified and the sensitivity of the assay will increase.
In diagnostics, polyclonal may also be useful due to their broader detection range. This allows them to effectively bind to the antibodies of patients from different ethnicities. Due to the natural genetic diversity of the human population, antibodies from different individuals often present very different glycosylation patterns and, thus, may not be recognized and captured by a single monoclonal antibody.
The biophysical diversity of these antibodies also makes then more resistant to environment conditions (i.e. temperature, pH, among others), which may cause the inactivation or precipitation of monoclonal antibodies. For this reason, they are also easier to store. Unlike monoclonal antibodies which often require the presence of stabilizing agents to prevent aggregation, precipitation and protect the binding affinity.
Overcoming the technical limitations of polyclonal antibodies
But, the enhanced sensitivity of these molecules comes with a price. For instance, these antibodies are more sensitive and robust, but their use increases the risk of cross-reactivity and high background signals.
For this reason, assays relying on the use of these molecules are often not recommended for analytical or therapeutic applications.
However, extra precautions can be taken into account to increase the standardization of assays using polyclonal antibodies.
For instance, due to the high batch-to-batch variabilities, it is desirable to include adequate positive and negative controls in each assay. Positive controls can be either native or recombinant antigen, or cells expressing the antigen. While cell with a gene KO may be used as negative controls. These controls have the double function of standardizing the results and allowing the determination of the real detection threshold.
The reduced specificity of polyclonal antibodies can also be reduced by performing extra antiserum purification steps. And the purified mixture can be tested against a panel of positive and negative controls (e.g. ELISA or WB) to further determine their real specificity.
Concluding remarks
The enhanced sensitivity and stability of polyclonal antibodies continues to harness the development of immunotechnologies for research applications.
Despite the high risk of cross-reactivity and decreased specificity of these molecules it is now possible to increase the robustness and standardization of these assays. This can be achieved by including additional selection steps to reduce unspecific binding and by including the proper positive and negative controls in each assay.
For these reasons, the pairing of monoclonal and polyclonal antibodies may be the ideal solution to detect large molecules, low-abundance targets or targets that are prone to antigenic drift or shift. Phenomena that are known to occur in fast mutating virus like influenza, that can swap entire regions of their genomes and reduce the binding affinity of a given antibody.
- Ascoli, C. A. and Aggeler, B. Overlooked benefits of using polyclonal antibodies. BIOTECHNIQUES. 2018; 65:3. doi: 10.2144/btn-2018-0065
- Berry, C. M. Antibody immunoprophylaxis and immunotherapy for influenza virus infection: Utilization of monoclonal or polyclonal antibodies? Hum Vaccin Immunother. 2018; 14(3): 796–799. doi: 10.1080/21645515.2017.1363135
