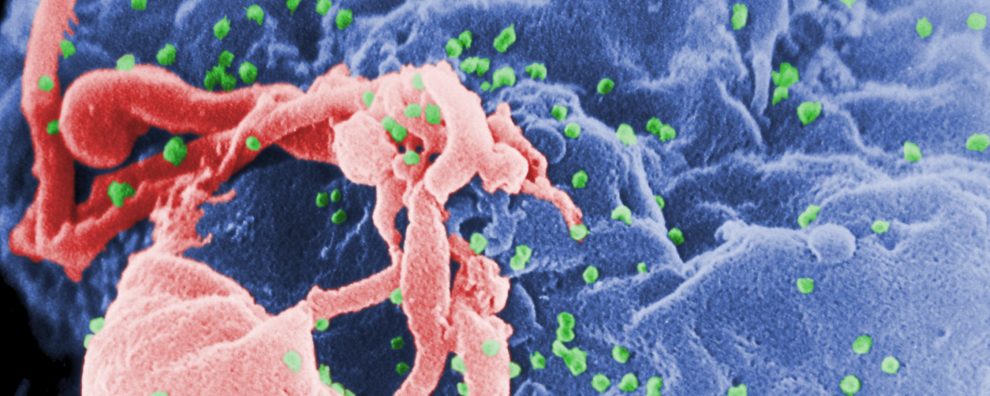
 Antibody production
Antibody production
How is antibody therapy changing the way we treat HIV?
The ever-elusive cure for human immunodeficiency virus (HIV) infection continues to be out of reach for most patients. But the latest developments promise to improve the current treatment options, bringing great hope to patients’ lifelong fight against the disease. As of March 2020, two very promising approaches are undergoing initial stages of preclinical and clinical evaluation: an HIV-neutralizing antibody cocktail and a monoclonal antibody blocking a key enzyme responsible for T cell deterioration. Through the first approach, scientists showed that neutralizing HIV viral particles resulted in the further engagement of the patient’s immune system. Through the second approach, by targeting a newly discovered enzyme responsible for the deterioration of blood cells in HIV-positive patients, scientists were able to arrest the progression of the disease. Both treatment options may forever change how we fight this disease.
To cure or to treat HIV?
Thirteen years after the successful cure of Timothy Brown, Adam Castillejo became the second patient ever to be freed of prolonged human immunodeficiency virus (HIV) infection. The retrovirus, that has swept the world since the early 1980s continues to afflict the lives of 37.9 million people. In 2018, HIV still was responsible for the deaths of 670,000 adults and 100,000 children around the globe.
Before Brown’s successful story, HIV infection had for long been considered a treatable but incurable illness. However, scientists urge us to be cautious, as these two patients represent a very specific group of individuals. In fact, Brown and Castillejo both had been diagnosed with aggressive forms of blood cancer in addition to the HIV infection. Thus, both needed a bone marrow transplant in addition to HIV treatment.
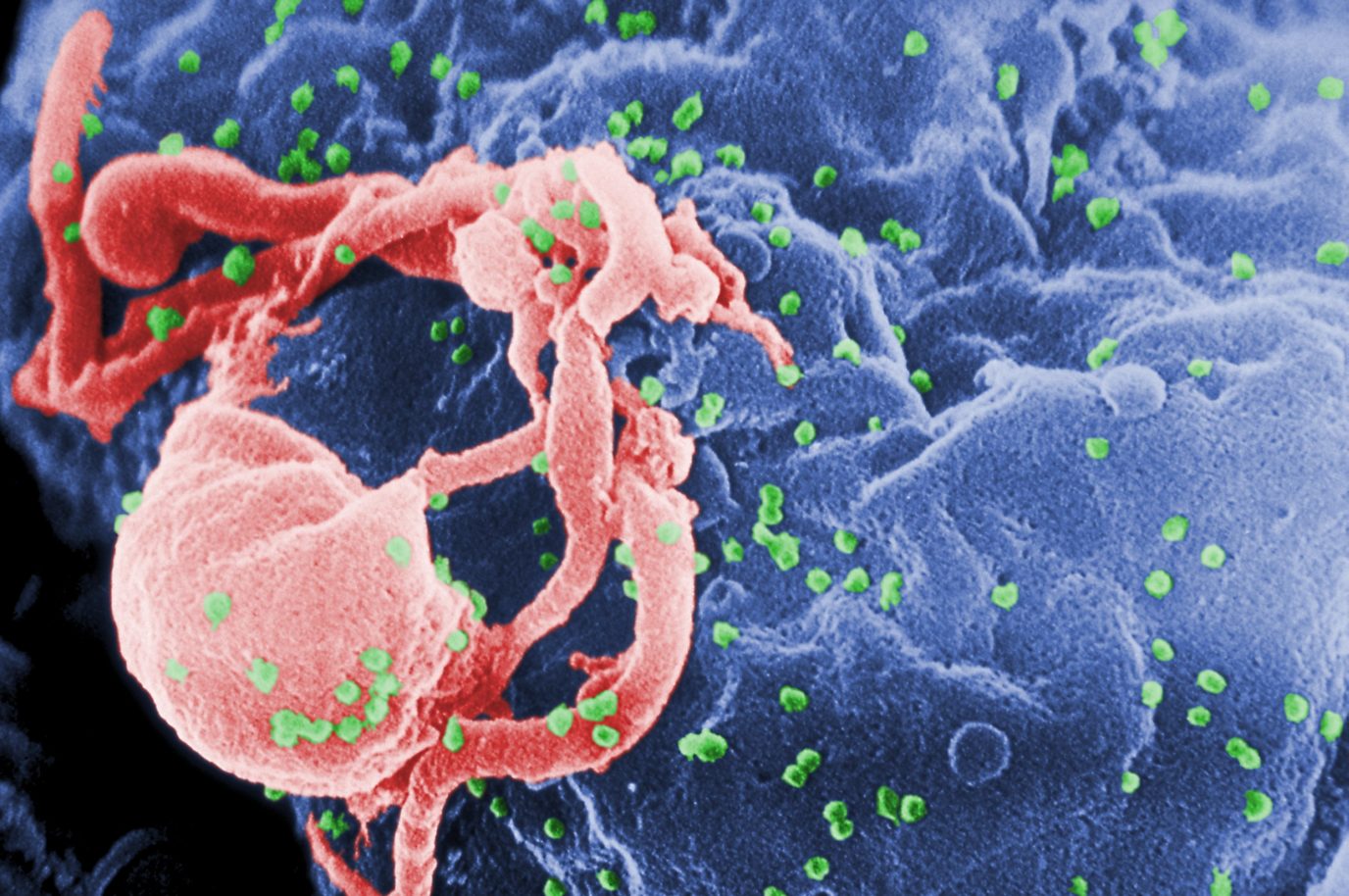
To prepare for the treatment, the blood cells of both patients were destroyed by chemo or radiotherapy, before they could be replenished with stem cells from a healthy donor. In their special case, the donors carried an extremely rare mutation in the CCR5 receptor, making them naturally immune to HIV. By transplanting these naturally immune cells, Brown and Castillejo were able to fight the infection and clear their organism from remaining viral particles.
However, a bone marrow transplant is an extremely delicate and complex procedure, sometimes resulting in fatal complications. For this reason, public health specialists consider the risk of the procedure to be far too great in comparison to conventional treatments and only recommend it when HIV-infected patients also need a bone marrow transplant.
But while the cure continues to be out of reach for most HIV-afflicted individuals, the improvement of current treatment options is close at hand.
Antiretroviral therapy (ART) and antibody therapy
For the past decades, antiretroviral therapy (ART) has been the treatment of choice for HIV-infected patients. For most patients, a combination of several of these drugs has been successfully used to keep the viral load to a minimum and delay the onset of the acquired immunodeficiency syndrome (AIDS) that develops during the later stages of the disease. Drugs used as part of ART consist of inhibitors targeting specific viral proteins such as integrase, nucleocapsid, capsid, and envelope proteins, among others.
For most patients, the intake of these drugs allows them to live a normal life. However, ART is not curative, and patients are required to take these drugs daily throughout their entire lives. Moreover, some patients still experience debilitating side effects such as nausea and vomiting, diarrhea, fatigue, pain, among others. Also, many patients inevitably develop resistance to one or more drugs used in conventional ART approaches.
Despite being highly successful at suppressing the virus, ART fails to activate the patients’ natural immune response by limiting the number of available antigens. Thus, it limits patients’ immune response to the invasive pathogen.
On the contrary, antibody therapy is known to carry fewer side effects than conventional ART, as antibodies act both as neutralization agents and as activators of the immune system.
The first anti-HIV antibody ever to be granted clinical approval in the US and the EU was Ibalizumab (Trogarzo™), in 2018 and 2019, respectively. Obtained and humanized from mouse, Ibalizumab targets the CD4 cell surface protein, the primary HIV receptor, and thus prevents the virus from infecting new cells. It was designed to be used in combination with ART and to help patients who can’t be treated with ART alone.
Currently, another monoclonal antibody – Leronlimab, or PRO 140 – is under regulatory revision and expected to be granted approval shortly. Leronlimab was also obtained and humanized from mouse and, in combination with ART, it protects healthy blood cells from HIV infection. But unlike its predecessor, it targets the CCR5 cell receptor instead of the CD4 receptor. According to CytoDyn, the company leading the development of this antibody, the feasibility of using Leronlimab as monotherapy is still under evaluation.
Anti-HIV antibody therapy
Interestingly, antibody therapies considered until now are still dependent on ART and have been designed to target surface cell receptors. But an international team of researchers from the University of Montreal Hospital Research Centre (CRCHUM), Rockefeller University (United States) and University of Cologne (Germany), has proposed another solution.
The team led by Daniel Kaufman showed that when patients were injected with a mixture of two strong anti-HIV neutralizing antibodies (3BNC117 and 10-1074) during ART interruption, their organism’s natural immune response kicked in resulting in a higher activity by CD4 and CD8 cells. Moreover, Kaufman and his team noted that all participants of this study maintained a viral suppression during at least 15 days after the treatment, suggesting that the injections wouldn’t need to be so frequent as current antiretroviral approaches.
This was the first study to show that anti-HIV antibodies, that target the virus itself, could produce a synergic effect by engaging the patients’ own immune system. However, the feasibility of using this form of therapy is still undergoing clinical trials.
New targets to prevent the onset of AIDS
Up to now, all antibody therapies have been focused on targeting well-established markers. But recently, a study published by Diaccurate has challenged these conventional approaches. In this study, the authors noted that almost 80% of CD4 cells in HIV-infected patients had morphological abnormalities.
These abnormalities consisted of numerous membrane microdomains (aMMDs) that trapped and inactivated cell receptors, making CD4 cells unresponsive to antigens and even contributing to their decline over time. But what caused these abnormalities?
The team found that PLA2G1B, a phospholipase A2 group IB enzyme, was the key driver of this process. The enzyme was found to bind HIV gp41 envelope protein, thus driving the enzyme to act on HIV-infected CD4 cells. By developing a murine monoclonal antibody that targets this enzyme (mAb 14G9), researchers obtained promising results both in vitro and in vivo using mice as models for the disease.
Diaccurate has recently announced that the humanized version of this antibody – Plazumab – is already undergoing preclinical evaluation.
Concluding remarks
Neither ART or current antibody therapies are curative, as they require the infection to be active to eliminate dormant HIV that creeps and hides in the genome of CD4 cells. These therapies fail to tackle what experts call the latent HIV reservoir. Unlike other viruses, HIV camouflages itself within healthy cells making its complete elimination very challenging.
Currently, several approaches to a “shock and kill” strategy are being evaluated for purging this latent HIV reservoir. Most strategies comprise two well-defined stages. The “shock” stage consists of using latency-reversing agents (LRAs) to activate latent provirus within CD4 cells, while the “kill” stage consists of using ART, antibody therapy, or a combination of the two to purge the remaining viral particles from the patient’s organism.
Experts believe these strategies will pave the way for a universal cure of HIV and significantly reduce the number of people who today still struggle with the disease.
- Awi, N. J. and Teow, S. Y. Antibody-Mediated Therapy against HIV/AIDS: Where Are We Standing Now? J Pathog. 2018; 2018:8724549. doi: 10.1155/2018/8724549
- Beccari, M. V. et al. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob Agents Chemother. 2019; 63(6):pii: e00110-19. doi: 10.1128/AAC.00110-19
- Centers for Disease Control and Prevention. HIV Basics. Retrieved from https://www.cdc.gov/hiv/basics/index.html Last accessed March 2020 Last accessed on March 2020
- (March 4, 2020). World Premiere: Diaccurate, The Biotech Created by Truffle Capital, Reveals the Mechanism of Immune Disablement in HIV Infection. Retrieved from https://www.businesswire.com/news/home/20200304005676/en/World-Premiere-Diaccurate-Biotech-Created-Truffle-Capital
- Niessl, J. et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med. 2020; 26(2): 222-227. doi: 10.1038/s41591-019-0747-1
- Pothlichet, J. et al. PLA2G1B is involved in CD4 anergy and CD4 lymphopenia in HIV-infected patients. J Clin Invest. 2020. doi: 10.1172/JCI131842
- Warren, M. Second patient free of HIV after stem-cell therapy. Nature News. March 3, 2020. doi: 10.1038/d41586-019-00798-3
- World Health Organization (WHO). Global Health Observatory (GHO) data. Summary of the global HIV epidemic (2018). Retrieved from https://www.who.int/gho/hiv/en/ Last accessed on March 2020
