 Antibody production
Antibody production
Tips to choose the best monoclonal antibody production process for your project
Early monoclonal antibody production processes relied on the use of immortalized hybridoma cell lines. However, these early production methods faced major drawbacks due to the low yields and genetic instability of these germlines. Over the years, due to advances in genetic sequencing, monoclonal antibody production has been engineered to improve the stability, scalability, and robustness of these processes. Check out other frequently asked questions (FAQs) page about monoclonal antibodies on our dedicated page.
Discovery and early development of monoclonal antibody production processes
Monoclonal antibodies (mAbs) are glycoproteins with the ability to recognize and bind to a single epitope (i.e. a single part) of an antigen. Due to their specificity, high affinity and ability to bind and agglutinate foreign molecules, mAbs have become useful and prompted the development of many areas of research, therapy and diagnostics.
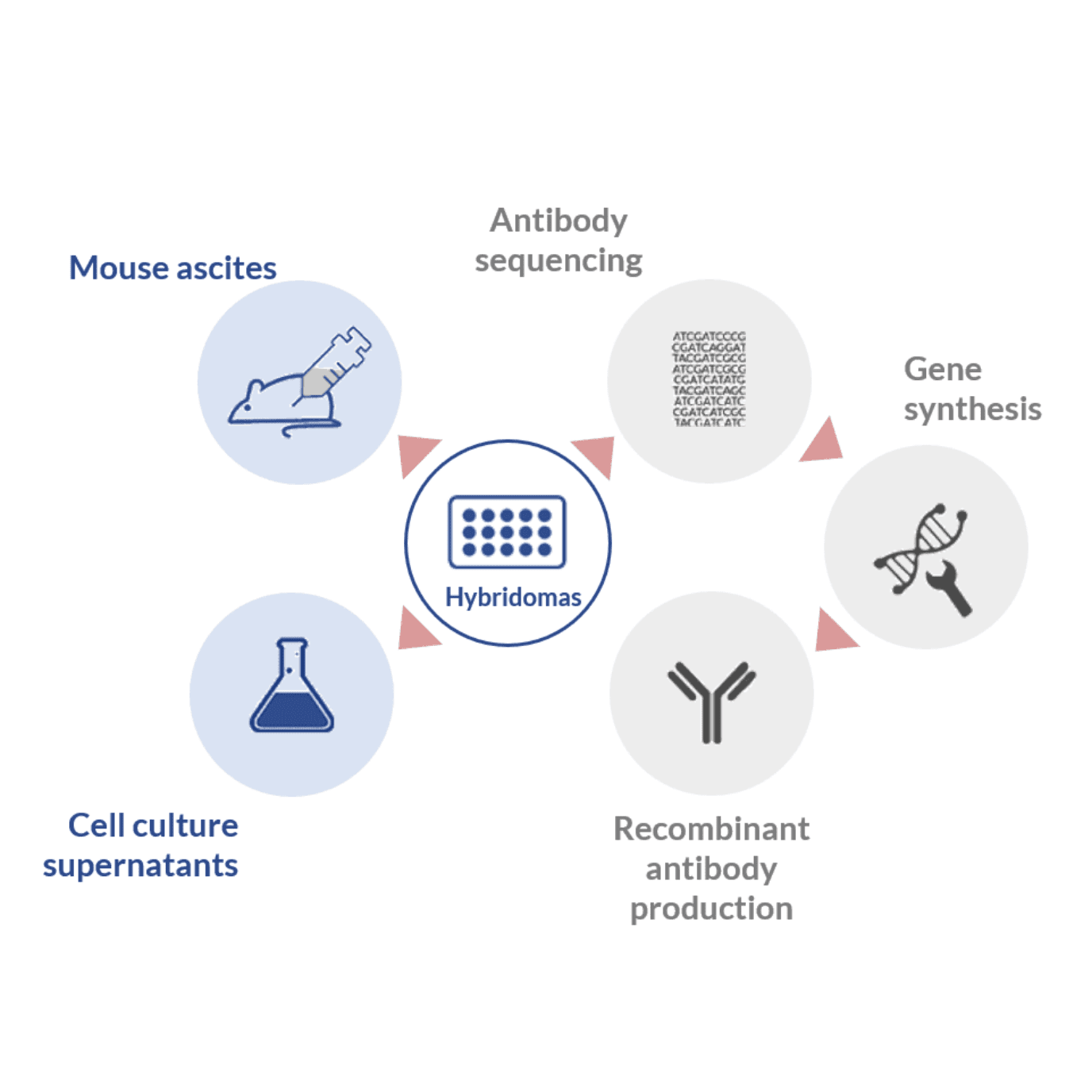
Historically, the production of mAbs was first accomplished in vitro through the use of hybridomas. These hybrid germlines resulted from the fusion of short-lived mature B cells harvested from immunized hosts with immortal myeloma cells, suited for in vitro culturing conditions.
This fusion, first obtained by Köhler and Milstein, was technically demanding and it presented many limitations. However, decades of research and technological development have allowed the establishment of more reliable, robust and mature cell culture technologies, able to support the large-scale production of monoclonal antibodies.
However, despite the progress, the hybridoma production technology still suffers from important drawbacks. For instance, it is well-known that some specific hybridoma cell lines fail to generate the required amounts of antibodies in vitro. Moreover, the recovery of antibodies from the cell culture medium and the downstream purification of these molecules often leads to denaturation and decreased antibody activity.
For these reasons, different monoclonal antibody production processes have been developed to compensate the inherent difficulties associated with the hybridoma technology.
Mouse ascites for monoclonal antibody production
The production of antibodies in vivo using mouse ascites was developed as an attempt to solve the problem of downstream purification and loss of affinity which resulted from in vitro culturing of hybridomas.
This method consists in the injection of stable hybridoma cell lines into the abdomen of a mouse. After injection this germline propagates in the peritoneal cavity for 1 to 2 weeks and cause the accumulation of a fluid containing large amounts of antibodies. This fluid, also called ascites or ascitic fluid, is usually collected over a series of “taps” in the peritoneal cavity (2.5 – 10 mg/ml unpurified yield) and easily purified using affinity purification methods for downstream applications.
However, due to the ethical implications of using animals for the production of antibodies and the availability of other in vitro methods, several countries have phased out the use of mouse ascites for antibody production (Germany, Netherlands, Austria, UK, Switzerland, USA, among others).
Presently, monoclonal antibody production processes relying in mouse ascites is strictly used for the production of antibodies that undergo significant changes during in vitro purification processes. A few examples of therapeutic antibodies that undergo significant denaturation during the in vitro purification process and are preferably produced through this method include:
- OKT3 – a mAb with substantial therapeutic application which cannot be adequately purified from culture fluids, so it must be produced by the ascites method.
- Immunoglobulin A (IgA) – in its monomeric form it has poor antigen-binding abilities. For this reason, this form must be separated from dimeric and polymeric IgA and this can be more easily accomplished using the mouse ascites method.
Cell culture supernatant method for monoclonal antibody production
Although a minority of hybridoma cell lines are unstable, the vast majority of antibody-producing hybridomas can be successfully grown in vitro in different culture systems: flasks, roller bottles (most common method), hollow fibers, wave bags and bioreactors. Furthermore, the culture medium can also be adapted to increase the purity and reduce the amount and quantity of potential contaminants in the final antibody preparations (i.e. medium with serum, serum-free medium or animal-free medium).
Technically, since this method does not rely on the use of animals, it is less labor intensive in comparison to mouse ascites. Its limitations include significantly lower yields (1 – 3 mg/ml unpurified antibody yields) in comparison to in vivo production in mice, and higher risks of loss of affinity during the concentration and purification process.
Recombinant antibody expression
In this method, the generation of highly stable and high-affinity hybridoma cell lines is followed by sequencing of the best-performing germlines. Sequencing allows the determination of the DNA sequence of the variable region or the full-length antibody. These sequences allow the construction, synthesis and cloning of the DNA of interest into the appropriate expression vector.
These vectors can be subsequently transfected into the desired expression system for large-scale production. The advantages of producing mAbs through recombinant processes is the possibility to use expression vectors with higher production yields than mouse ascites or hybridoma culture supernatant (in the g/l range). Furthermore, this process allows to fine-tune the affinity of the monoclonal antibody and the production of highly stable cell lines.
Several expression systems are currently available including many different chinese hamster ovary (CHO) cell lines (CHO-K1, CHO-DG44), mouse myeloma (NS0), baby hamster kidney (BHK), and human embryonic kidney lines (HEK293).
Traditionally, mammalian cell lines are the preferred expression systems for the production of recombinant monoclonal antibodies. The use of mammalian cell lines offers the unique advantage of preserving the glycosylation pattern of antibodies and, for this reason, decrease the risk of immunogenicity.
However, in recent years several other expression systems are being adapted and engineered for the production of therapeutic antibodies. For instance, the glycosylation-engineered cell lines from yeast, insects, and transgenic plants have been successfully used for the production of antibodies with “human-like” glycosylation patterns.
Concluding remarks
Presently, with the development and engineering of different cell lines, expression systems and optimization of in vitro antibody production processes, it’s increasingly easier to produce large amounts of high-affinity antibodies without the need to use animals in any stage of the process.
In this context, recombinant monoclonal antibody production processes comprise the most robust and reproducible way to produce large amounts of high-affinity and high-purity antibodies for therapeutic applications.
- Liu, J. K. H. The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann Med Surg (Lond). 2014; 3(4): 113–116. doi: 10.1016/j.amsu.2014.09.001
- Köhler, G., Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256:495-497. doi:10.1038/256495a0
- Frenzel, A. et al. Expression of Recombinant Antibodies. Front Immunol. 2013;4:217. doi: 10.3389/fimmu.2013.00217
- Kunert R. and Reinhart D. Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol. 2016; 100: 3451–3461. doi: 10.1007/s00253-016-7388-9
- National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies. Monoclonal Antibody Production. Washington (DC): National Academies Press (US); 1999. 3, Scientific Needs for Mouse Ascites Production of mAb. Available from: https://www.ncbi.nlm.nih.gov/books/NBK100195/.
- Wurm, F. M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393-8. doi: 10.1038/nbt1026
