 Antibody production
Antibody production
Why you should consider developing a humanized antibody for therapeutic applications
Early antibody therapy relied on antibodies developed from mouse cell lines. However, due to the low complementarity of these molecules to patient’s immune system, it became urgent to develop new methodologies to adapt these antibodies for therapeutic applications. Today, the creation of humanized antibodies from molecules of non-human origin, remains one of the most important processes for therapeutic drug development.
Early challenges in the development of therapeutic antibodies
Antibodies are complex Y-shaped glycoproteins with the ability to bind to specific foreign particles, also known as antigens. The potential of these molecules for therapy and diagnosis was recognized early on by researchers, leading to the laboratory-scale production of the first monoclonal antibodies from mouse cell lines in 1975 by Georges Köhler and César Milstein. This process became known as the hybridoma technology and, to this day, it remains the gold-standard of antibody generation.
However, the initial excitement instigated by the potential of these molecules was short-lived, as researchers realized the limitations of using non-human antibodies for clinical application. The administration of these antibodies from xenogeneic sources in human patients often led to immunogenicity, which neutralized the efficiency of these molecules and precluded their repeated use.
This response reduced the half-life of these molecules by quickly eliminating these antibodies from patient’s plasma; a reaction that later became known as the human anti-mouse antibody (HAMA) response. Moreover, these antibodies, also known as murine antibodies, were also found to have short half-lives in the patient’s plasma presumably due to their lower affinity towards human receptors, further hindering their application.
Chimerization – the first step in the development of humanized antibodies
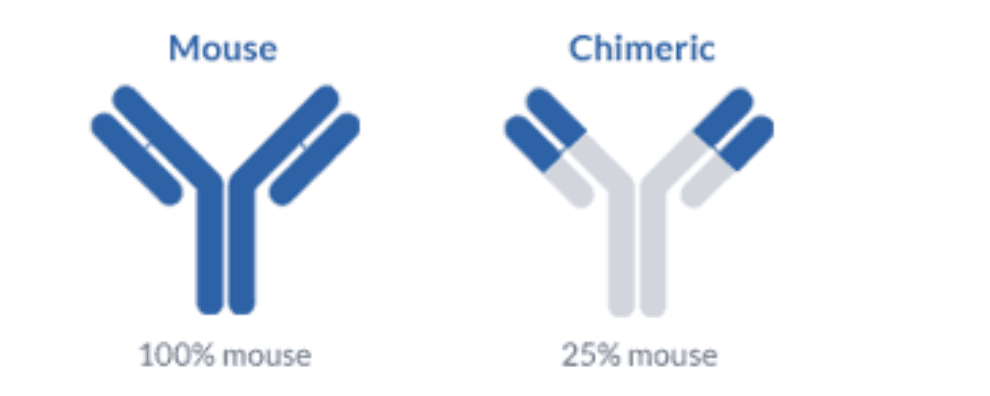
These limitations prompt the development of several antibody engineering techniques with the aim of reducing the HAMA response, resulting in the creation of chimeric and humanized antibodies. Chimeric antibodies represented the first breakthrough in the effort to increase the efficiency and feasibility of antibody therapy. This technique consisted in the synthesis of mosaic antibodies containing the variable domains of the mouse molecule and the constant domains of the human molecule.
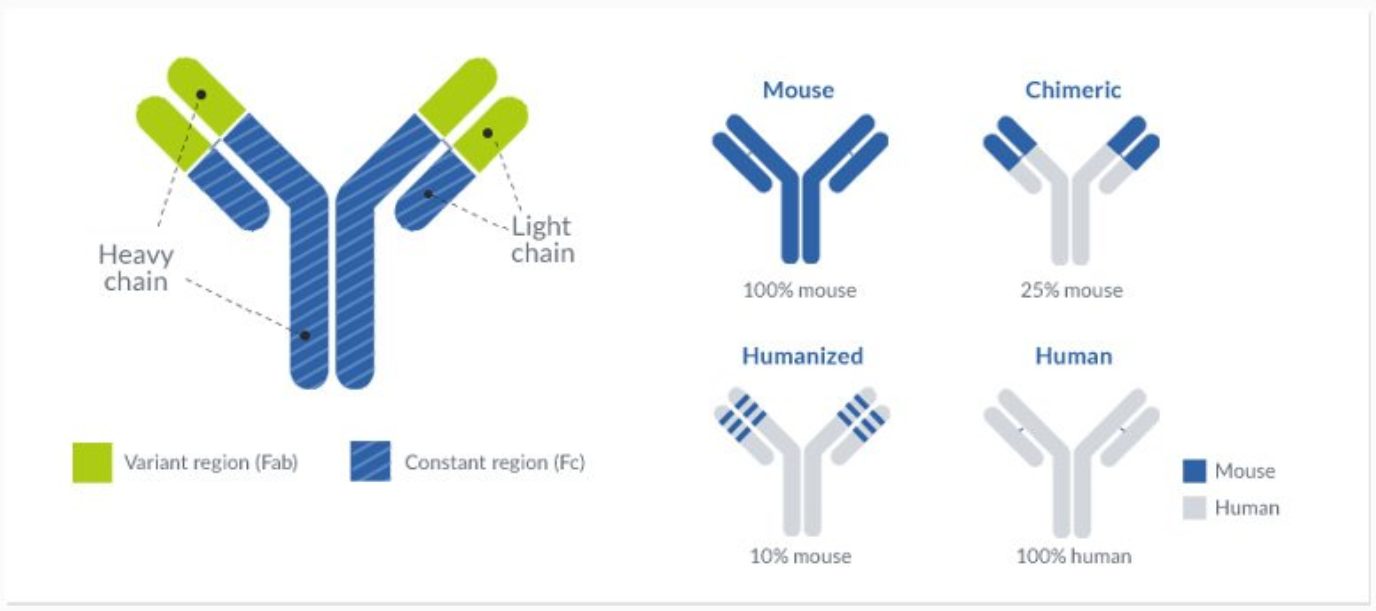
Chimerization resulted in the synthesis of antibodies that were 70% human and allowed them to interact more effectively with human effector cells and the complement cascade. The increase in sequence homology to human antibodies resulted in a significant reduction of the HAMA response in patients while maintaining the binding specificity of the glycoprotein and increasing the half-life of these drugs.
The first humanized antibody obtained by this technique was Daclizumab. It targeted the CD25 (Interleukin-2 receptor alpha chain) and was approved for therapeutic use in 1997. This therapeutic agent was developed from the mouse antibody ‘anti-Tac’ by using the human mature antibody EU as framework acceptor sequences (FR scaffolds).
However, clinical trials revealed that several of these humanized antibodies still elicited a modest immunogenic response in some patients, limiting their use for therapy. In a further effort to eliminate the HAMA response, researchers devised a strategy to increase the homology of the engineered antibody through a process that was later named antibody humanization.
Humanized antibodies obtained by CDR grafting
The humanized antibody differed significantly from the chimeric antibody because it was created through the selective modification of the mouse variable domain. These modifications were possible since only one-fourth of the amino acid residues of the variable domain play a role in the antigen’s binding. The resulting antibodies could be modified to increase homology to the human glycoproteins without completely compromising its binding efficiency and selectivity.
This technique was named CDR (complementary determining regions) grafting, and it created humanized antibodies that were 85-90% human and less immunogenic than its chimeric counterparts. However, this process revealed to be more technically demanding than the mere fusion of the variable and constant domains of antibodies from different origins.
CDR grafting often resulted in a reduced affinity of the protein towards its original target. This occurs because some residues, although not directly linked with antigen binding, play an important role in supporting the conformation of the CDR loops and in stabilizing the interaction of the heavy (VH) and light (VL) chains of the variable domain.
To circumvent the problems created by the CDR grafting technique, it became crucial to carefully plan and design these molecules with the aid of computer modeling techniques and sequence optimization. This optimization step is therefore crucial to maintain the affinity and specificity of the mosaic antibody towards the target, while also minimizing the immunogenic response in patients.
Humanization allowed the development of many monoclonal antibodies for application in therapy. Although more advanced and complex technologies are available (e.g. phage display technologies), CDR grafting together with modeling and optimization remains one of the most popular and widely preferred techniques for the development of new treatments due to its high effectiveness and cost-benefit.
Humanized antibodies in therapy
Presently, more than 80 antibodies have been approved for therapy by the Food and Drug Administration (FDA, US) and by the European Medicines Agency (EMA).
In 2018 alone, a record number of antibodies have been approved by these two agencies (12). Half of these were humanized antibodies (fremanezumab, caplacizumab, galcanezumab, mogamulizumab, ibalizumab and tildrakizumab) while the other half originated from human sources. These antibodies are now recurrently applied for the treatment of cancer, autoimmune and genetic diseases, asthma, cardiovascular and hematologic diseases, and macular degeneration, among others.
This data shows that antibody humanization is an increasingly popular technique for the development of therapeutic antibodies. Moreover, antibodies developed through this technique have an equal probability of being approved for therapy in comparison to human antibodies.
- Santos, M. L., Quintilio, W., Manieri, Manieri, T. M., Tsuruta. L. R., Moro, A. M. Advances and challenges in therapeutic monoclonal antibodies drug development. Brazilian J. Pharm. Sci. 2018; 54:e01007. doi:10.1590/s2175-97902018000001007.
- Mayrhofer, P., Kunert, R. Nomenclature of humanized mAbs: Early concepts, current challenges and future perspectives. Hum. Antibodies. 2018; 27(1): 37–51. doi:10.3233/hab-180347.
- Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S., Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986; 321(6069):522-525. doi: 10.1038/321522a0.
- Kaplon, H., Reichert, J. M. Antibodies to watch in 2019. MAbs. 2019; 11(2):219-238. doi: 10.1080/19420862.2018.1556465.
- Köhler, G., Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256:495–497. doi:10.1038/321522a0.
- Williams, D. G., Matthews, D. J., Jones, T. Humanising Antibodies by CDR Grafting. in Antibody Engineering. Springer, Berlin, Heidelberg. 2010; pp 319–339. doi:10.1007/978-3-642-01144-3_21.
- Safdari, Y., Farajnia, S., Asgharzadeh, M., Khalili, M. Antibody humanization methods – a review and update. Biotechnol. Genet. Eng. Rev. 2013; 29:175–186. doi:10.1080/02648725.2013.801235.
- Chames, P., Van Regenmortel, M., Weiss, E., Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br J Pharmacol. 2009; 157(2):220–233. doi:10.1111/j.1476-5381.2009.00190.x.