 Antibody production
Antibody production
The role of point-of-care tests in tracking and curbing the COVID-19 pandemic
Coordinated strategies, efficient tracking, and vaccination are key to ensure a safe return to normal during the COVID-19 pandemic. But as regulators and policymakers work at record speed to implement efficient vaccination strategies around the globe, tracking the disease will be more important than ever, especially now that seasonal influenza is bound to spread alongside SARS-CoV-2.
What are point-of-care tests?
Since the development and commercialization of RT-qPCR tests to detect SARS-CoV-2, many other methods have been created and are currently being implemented at specific locations. This second generation of COVID-19 diagnostic tests focuses on rapid analysis and simplicity, allowing researchers and clinicians to cover a vast area and population and deterring potential clusters before they have the chance to spread.
Collectively, these are known as point-of-care (POC) tests. POCs possess rapid turnaround times and can typically be performed at specific settings such as pharmacies, urgent care facilities, clinics, nursing homes, and even temporary locations like drive-through sites or airports. These tests require only a sample of blood, urine, saliva, or other bodily fluid to provide a rapid and differential diagnostic without relying on expensive equipment.
POC tests present other important advantages over conventional PCR, such as the ability to diagnose patients at their point of treatment or even at their own homes, protecting both risk groups and frontline healthcare personnel. Many FDA-authorized POC tests are currently in use to detect SARS-CoV-2 consisting of medical portable devices based on chromatography, ELISA, fast molecular tests (e.g., Cepheid’s Xpert® Xpress SARS-CoV-2 test), or the most recent breath-analysis based on gas chromatography-ion mobility spectrometry (GC-IMS).
Types of point-of-care tests to track the COVID-19 disease
- RT-qPCR kits
The most abundant type of POC tests consists of RT-qPCR-based diagnostics taking nasopharyngeal and oropharyngeal swabs as samples. Many of these assays are multiplexed, targeting several regions of the virus, with enhanced assay precision and robustness.
Unlike other POC, RT-qPCR requires several hours to produce results, consumes expensive reagents, and requires specialized equipment. Interestingly, PCR results are also time-dependent, as threshold concentrations of the virus are necessary for accurate detection (i.e., convalescent patients often produce negative results). Plus, studies show that the virus is not evenly distributed in the respiratory system, often producing false-negative results when samples are incorrectly collected.
For these reasons, although PCR remains the dominant technology when it comes to SARS-CoV-2 diagnostics, other test formats have been gaining importance in the design of effective pandemic management strategies.
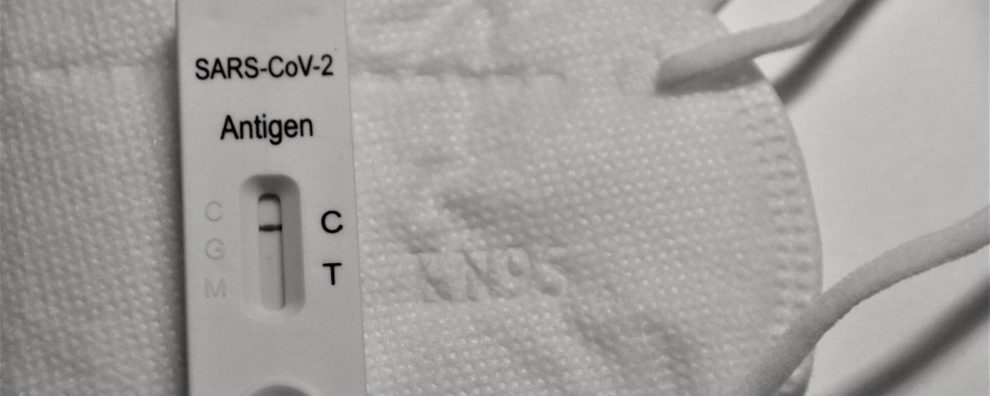
- Lateral flow chromatographic immunoassays (LFIA)
Taking blood, plasma, or saliva samples, these tests are designed to detect the presence of anti-SARS-CoV-2 antibodies (IgG/IgM) or viral antigens. LFIA works similarly to ELISA in the sense that a labeled antigen/antibody is used to capture the target, producing a signal as a result. However, ELISA requires specialized equipment for assay preparation and analysis, while LFIA is based on visual evaluation and disposable portable devices.
This difference stems from the assay format and the specific label used in the two different assays. In ELISA, the label is enzymatic, requiring a substrate to produce a signal (color change). In contrast, LFIA can employ different types of stable and inexpensive labels including colloidal gold nanoparticles, colored latex, magnetic and carbon nanoparticles, quantum dots, phosphors, fluorophores, among others.
For COVID-19, most commercially available LFIA tests detect IgG/IgM antibodies with the help of colloidal gold nanoparticles-labeled antigens in conjugation with anti-human IgG/IgM antibodies to differentiate the two anti-SARS-CoV-2 antibody isotypes present in the patient’s blood.
Despite being slightly more expensive than conventional ELISA, LFIA presents important clinical advances, such as the possibility of performing tests without specialized equipment including at airports, hospitals, and faster turnaround times. In the IgG/IgM detection format, LFIA allows the detection of the immune state of individuals (seroconversion) by indicating contact with the pathogen and the strength of his/her immune response. These tests are typically used in population-wide screening and as a preliminary diagnostics tool. Moreover, these tests could also help regulators and policymakers define priority groups for vaccination.
In summary, the attractiveness of these portable tests in comparison to RT-qPCR stems from their ease of use (no extensive training required), simple storage conditions (room temperature), high analytical sensitivity, selectivity, and straightforward interpretation of results (visual evaluation of bands).
- Breath-analysis
Based on the detection of specific markers by GC-IMS, this methodology allows a quick (<10 min) differentiation of COVID-19 from influenza patients or others suffering from different respiratory infectious diseases. Breath-analysis has been considered by some experts as a valuable alternative to other POC tests since the sample collection process is non-invasive – patients have only to exhale through a single-use disposable polypropylene tube.
The comparative breath analysis between COVID-19 patients and others suffering from different respiratory diseases, identified aldehydes (ethanal, octanal), ketones (acetone, butanone), and methanol as the signature volatile organic compounds in the COVID-19 disease with an overall accuracy above 80%.
Concluding remarks
POC tests are invaluable tools for the fast-tracking of the COVID-19 pandemic. Despite RT-qPCR still being the dominant test format, other test types have since become available. In the current context, LFIA and breath analysis continue to gain ground in COVID-19 diagnostics at point of care locations.
They present important advantages over conventional RT-qPCR assays including the ease of execution and fast turnaround times. LFIA is an important alternative since it is easy to perform (no special training required), doesn’t require specialized equipment, and the results are easy to interpret. In contrast, breath-analysis has recently emerged as a suitable method to differentiate between seasonal flu and COVID-19.
Despite the availability of COVID-19 vaccines, tracking the spread of the virus and the development of immune responses (seroconversion) in the population will still be necessary. On the one hand, these tests will be essential to determine the effects of the vaccine and identify priority groups within the population. On the other hand, these tests will help to protect the groups that won’t be able to receive the COVID-19 vaccine: children, immunocompromised individuals, pregnant women, and people suffering from allergies.
- Andryukov. B. G. Six decades of lateral flow immunoassay: from determining metabolic markers to diagnosing COVID-19. AIMS Microbiology. 2020; 6(3): 280-304. doi: 10.3934/microbiol.2020018
- Ruszkiewicz, D. M. et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry – a feasibility study. EClinicalMedicine. 2020; 100609. doi: 10.1016/j.eclinm.2020.100609
