 Antibody production
Antibody production
What stimulates antibody generation and how to leverage it for the design of effective immunization strategies
Understanding what stimulates antibody production is important to improve the efficiency of both active and passive immunization strategies. Due to the complexity of the human immune response, it is not only important to understand what types of molecules activate the immune system, but also, what types of cells and molecules coordinate and regulate antibody expression. Check our frequently asked questions (FAQs) page about hybridomas for a complete overview of all steps of this robust process for antibody generation.
- Molecules and cells involved in the immune response
- Antigen-presenting cells (APC) and their role in the immune response
- Immune repertoires and the onset of personalized immunization strategies
- What stimulates antibody generation when small immunogenic molecules are used as antigens?
- Concluding remarks
Molecules and cells involved in the immune response
In our organism, antibodies are produced in response to foreign molecules, called antigens. The natural adaptive immune response can generate antibodies capable of recognizing a multitude of different molecules including proteins, carbohydrates, and lipids.
The adaptive response can happen through two different processes: T cell-dependent or T cell-independent process (TD or TI, respectively). The activation of each of these processes depends on the nature of the antigen. For instance, most molecules and pathogens need to be digested and processed before being able to cause an effective immune response. But large polymeric molecules are often sufficient to activate the quick TI response in adults.
The inherent limitation of the TI response arises from its inability to produce an immunogenic memory. This primary response requires only the interaction between a soluble antigen and a B cell with compatible receptors (BCR) and can quickly produce antigen-specific antibodies. However, most protective and active immunization strategies (i.e. vaccines) tend to rely on the more complex and stronger TD immune response.
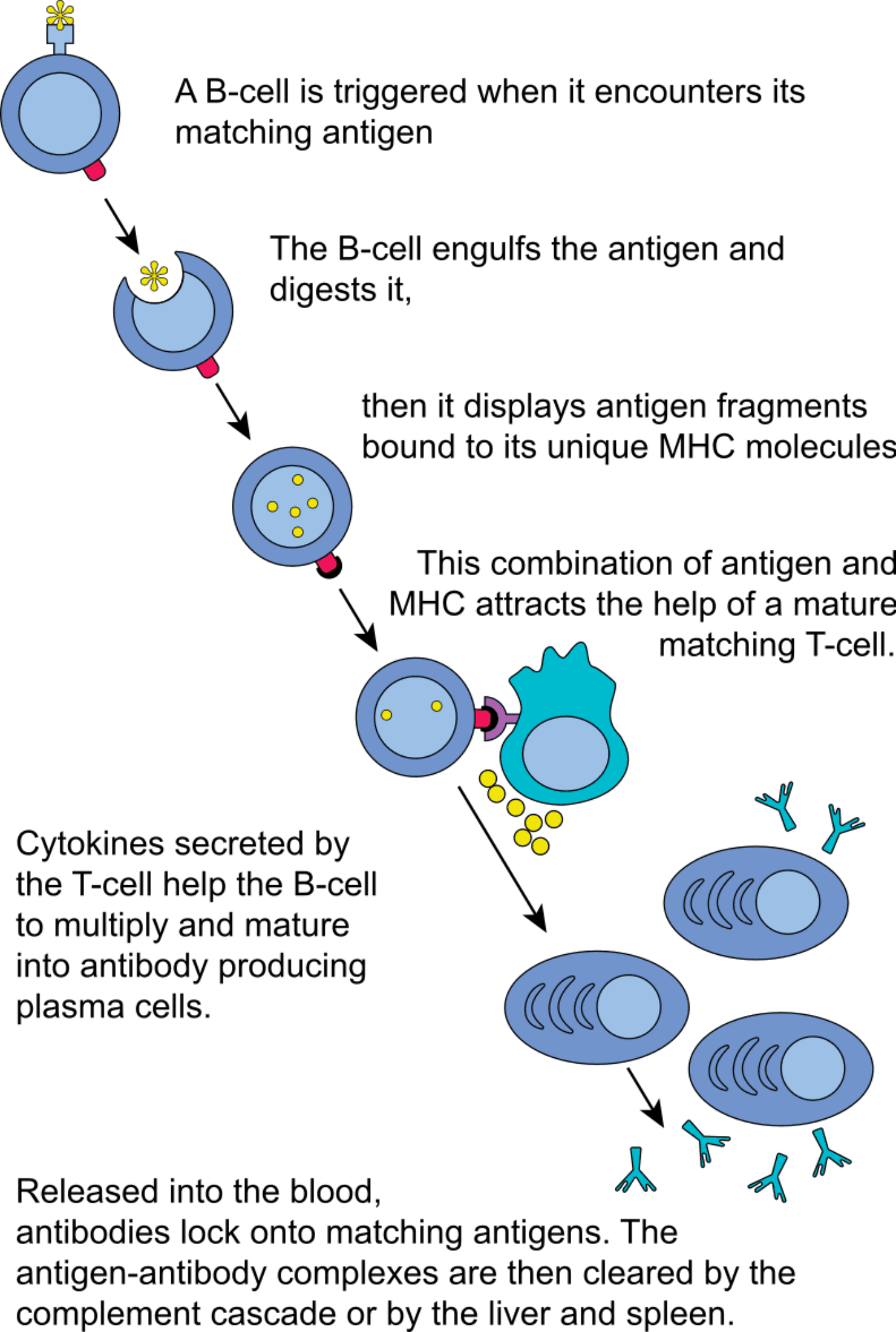
In this case, the diversity of antigens able to stimulate antibody production in our organism increases exponentially. This happens because, unlike B cells, T cells are not activated by soluble antigens. Instead, T cells need to interact with professional antigen-presenting cells (APC), such as dendritic cells, before the immune response can be initiated.
Antigen-presenting cells (APC) and their role in the immune response
Before naïve T cell activation can occur, these cells need to be activated by specialized APC. Notoriously, APC are responsible for degrading foreign molecules and microorganisms and complexing their fragments with MHC (major histocompatibility complex) molecules.
T cells responsible for interacting with antibody-producing B cells, express the CD4 receptor and are named helper T cells or CD4+ T cells. Via their T-cell receptor (TCR), these cells recognize only the foreign fragments that are bound to class II MHC molecules on the surface of APC.
One limitation of this mechanism occurs due to the natural bias of T cells. The proliferation and recombination of the genetic machinery of these cells are heavily regulated to avoid immunopathology. For this reason, the efficiency of the TD response also depends on the organism’s previous infections with the same or similar pathogens. This complicates the TD response to a completely new pathogen. In the case the organism has never encountered a similar antigen, it needs to produce large variations of precursor cells before obtaining compatible TCRs.
TCRs recombination is an extremely and tightly regulated process, however, it is also crucial for increasing the diversity of CDRs (complementary determining regions). CDRs, both in TCRs and B cell receptors (BCRs), are responsible for antigen interaction. For this reason, a high diversity of CDRs ensures the organism better and more effective protection against pathogens. In other words, the more a single organism is exposed to different pathogens and foreign molecules, the better equipped it becomes to deal with new threats.
Immune repertoires and the onset of personalized immunization strategies
This knowledge challenges the assumption that healthy individuals have unlimited antigen-recognition repertoires. Moreover, this constraint of naïve repertoires may limit the efficiency of conventional vaccination strategies.
This limitation has prompted many researchers to investigate personalized vaccination as a suitable alternative to conventional strategies. According to experts on the topic, vaccines may be tailored to specific groups of individuals (defined by common characteristics such as sex, age, ethnicity, Fc-gamma-receptor polymorphism, immune status, etc.) to specifically engage with their unique immune repertoires.
However, the personalization of vaccines comprises challenges with many different levels of complexity. Moreover, in many cases, the increase in efficiency may not compensate for the additional cost of developing different vaccine formulations according to each group’s specific needs. The main reason for this significant increase in cost is the lack of extensive studies on the immune repertoire of individuals from specific groups. Thus, as stated by Katja Fink, a scientist from the Singapore Immunology Network, there is an urgent need to increase our repertoire databases before the field can move forward.
What stimulates antibody generation when small immunogenic molecules are used as antigens?
Low molecular weight molecules (<1,000 DA) are often not naturally immunogenic. Hence, they cannot be directly used to stimulate antibody production in our organism. In these cases, effective active immunization strategies can be created by conjugating the small molecule with a larger protein. It is the complex, not the hapten itself, that stimulates antibody production.
This process is called the anti-hapten response. Haptens are thus considered molecules that only elicit an immune response when linked to a macromolecule, also known as carrier. Nevertheless, haptens are a structurally and chemically diverse group, thus making the process of hapten conjugate design very complex from a chemical point-of-view.
When a hapten is used for immunization, special consideration must be given to the coupling strategy and the hapten:carrier molar ratio. These are the two factors that directly influence the strength of the immune response.
Many proteins have been successfully employed as carriers, the most frequent include globulins and albumins, gelatin, casein, and tetanus, cholera or diphtheria toxoids. The only requirements of a good carrier molecule include natural immunogenicity and the presence of sufficient reactive side chains that can be leveraged for conjugation with the hapten.
Conjugation strategies are also diverse. For instance, when the hapten is chemically reactive, the conjugation can be spontaneous. However, when natural reactivity is weak, haptens may be conjugated by crosslinking with intermediary molecules such as carbodiimides or glutaraldehyde.
Concluding remarks
In our organism, the strongest immune responses are TD. The need for cell-cell interaction between B cells, T cells, and dendritic cells, makes this process extremely complex. From the point of view of designing better active and passive immunization strategies, it is important to understand what stimulates antibody production in vivo.
Polymeric substances are often sufficient to elicit an effective immune response in adults. However, more complex antigens need to take into account the need for T cell activation by dendritic phagocytes. Moreover, small molecule antigens (haptens) often fail to elicit any type of immune response and need to be conjugated to large carriers to ensure T cell activation.
Interestingly, the effectiveness of an immunization approach also depends on the naïve repertoire of each individual. For this reason, many researchers have called out for the need to develop personalized immunization strategies, taking into account the patient’s age group, immune status, and other important factors.
Nevertheless, the response of individuals from different groups to vaccines and antibody therapies is still scarce. For this reason, a step forward towards personalized medicine needs to start with extensive studies and database generation regarding how each patient reacts to a given therapy.
- Clambey, E. T. et al. Molecules in medicine mini review: the αβ T cell receptor. J Mol Med (Berl). 2014; 92(7): 735–741. doi: 10.1007/s00109-014-1145-2
- Lemus, R. and Karol, M. H. Conjugation of haptens. Methods Mol Med. 2008; 138:167-182. doi: 10.1007/978-1-59745-366-0_14
- Mayer, A. et al. Regulation of T cell expansion by antigen presentation dynamics. Proc Natl Acad Sci U S A. 2019; 116(13):5914-5919. doi: 10.1073/pnas.1812800116
- Sprent, J. Antigen-Presenting Cells: Professionals and amateurs. Curr Biol. 1995; 5(10):1095-1097. doi: 10.1016/s0960-9822(95)00219-3
