 Antibody production
Antibody production
Why humanized antibody production may thrive in the 2020s
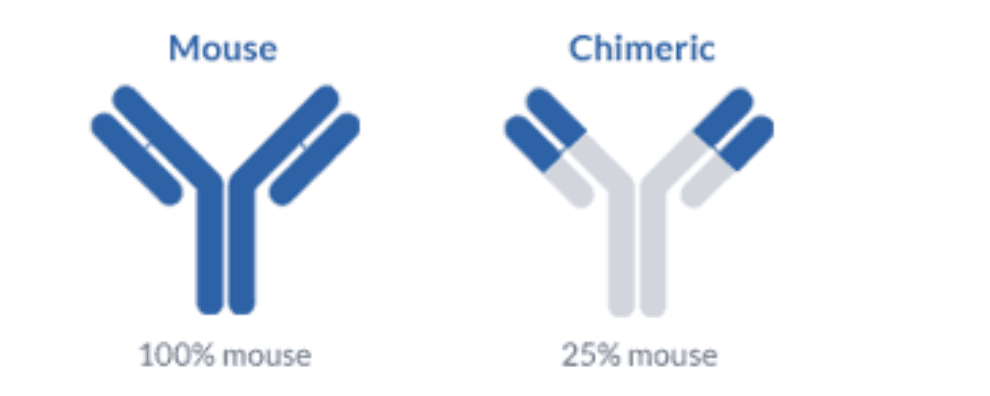
For the first time, humanized and fully human antibodies are almost on equal terms. Up to 2019, 38 humanized antibodies were granted clinical approval, whilst 30 human antibodies have followed on its tracks. Interestingly, all antibodies approved in 2019 were obtained by humanizing immunoglobulins derived from mouse and rabbit.
What does this trend tell us about humanized antibody production?
Many experts seem to agree that the focus of immunotherapy development will shift from antibody humanization towards the faster development of human antibodies. However, the discovery of the extremely versatile rabbit and camelid antibodies may be leading us to rediscover the value of the antibody humanization technique.
Contrary to human antibodies, which can currently be obtained in shorter timeframes, humanized antibodies often require longer developmental times. This constraint derives from the need to “humanize” the antibody sequences obtained through the hybridoma technology or the phage display platform.
On the current landscape, the process of humanization requires an optimal combination of in silico sequence optimization and the ability to quickly produce a vast number of “humanized” variants. It’s in the recombinant production that this technique still faces its greatest challenges.
The reason for these constraints arises from the need to use recombinant mammalian systems even during these early stages. These systems, even when employing high-performing transient expression approaches, still pose considerable time delays. Moreover, even the most optimal in silico design strategies may not translate to molecules with high in vitro activity, and many humanized antibodies need to undergo additional processes of affinity maturation to compensate for losses resulting from the humanization process.
Is the extra developmental bottleneck worth it?
Considering these technical constraints, it is natural to wonder if these techniques will still be relevant and useful in the next decades. Nevertheless, the 2020s may present unique opportunities for reinventing this technique in light of recent technological and scientific progress.
Decades of research inform us that humanization is still an invaluable technique to reduce the immunogenicity and ensure the therapeutic efficiency of xenogeneic antibodies. Moreover, the recent success of Brolucizumab, a rabbit-derived antibody, shows us that the technique that was developed to convert murine antibodies can be easily translated and applied to antibodies obtained from other species.
This can be considered one of the main advantages of humanized antibody production. Its ability to adapt any molecule, from any host to the desired humanized format. Moreover, since rabbit and camelid antibodies possess an increased affinity due to the different configuration of their CDRs, it may be extremely advantageous to develop these molecules for clinical applications.
Other experts point out the current limitations of current human antibody discovery technologies. Currently, human antibodies can be obtained from screening high-diversity human antibody libraries through display techniques (e.g. phage display) or through the immunization and hybridoma development using transgenic humanized mice as hosts. Both techniques present unique limitations in the face of antibody humanization from xenogeneic hosts.
For instance, mice, even humanized mice, have been shown to respond weakly to certain types of human antigens. Moreover, its usability with toxic antigens is quite limited. When it comes to phage display from human libraries, the approach requires the use of extremely high diversity libraries, that may have led to the discovery of only weak binders to a given antigen. For this reason, many human antibodies obtained from the phage display technology often need to undergo the time-consuming process of affinity maturation before they achieve the desired in vitro affinity. A limitation shared with current antibody humanization techniques, which often result in significant losses of affinity that need to be compensated through antibody engineering.
Current approaches to humanized antibody production
Although several techniques have been developed to humanize xenogeneic antibodies, is still difficult to assess the best performing technique. This difficulty in determining the efficiency of a given humanization strategy stems from the fact that the real immunogenicity of these humanized variants is hard and costly to assess. Often humanized antibodies that perform well on preclinical toxicity tests, may cause adverse and unexpected reactions during clinical trials.
Despite this limitation in terms of data, the most commonly used technique that has resulted in many clinical approvals is the CDR-grafting. The underlying principle of the technique is very straightforward. It implies the grafting of the CDRs (the regions responsible for the interaction with the antigen) and integration into a human antibody framework (i.e. the FRs responsible for scaffolding).
These simple act of extricating the binding sequences from the xenogeneic framework and replacing it with a human framework is sufficient to increase the homology of these molecules and, thus, reduce its immunogenicity. However, this process can cause a partial or complete loss of antigen-binding affinity, due to potential changes in the conformation of the original CDRs.
This loss led to the development of in silico tools to aid in the modulation and identification of key residues in the original molecule. Currently, these techniques based on structure reconstruction and in silico prediction algorithms have helped to alleviate the constraint caused by the need to produce many humanized xenogeneic antibodies in order to assess their antigen-binding affinity.
Due to the success of these approaches, humanized antibody production approaches will likely strongly rely on the development of more accurate design and modeling approaches. These methodologies should thus focus on developing more accurate predictions and therefore reduce the number of necessary variants that need to be produced and tested in vitro.
Since most protein structure prediction algorithms rely on the availability of experimental data, the improvement of current prediction models will likely occur by intensifying the structural elucidation of antibodies. A characterization that can be achieved through the use of high-resolution techniques such as NMR, X-ray crystallography, and cryo-EM techniques, among others.
Concluding remarks
Although human antibodies continue to gain ground over their humanized counterparts, humanized antibody production may continue to be an invaluable technique. Moreover, the recent approval of Brolucizumab may have renewed the interest of researchers and industry leaders in this technology.
Several factors contribute to making this technique advantageous in the face of current human antibody discovery techniques. For instance, humanization allows the generation of antibodies with no restriction of host nor antigens, moreover, humanized antibodies have a strong track record of clinical efficiency.
Nevertheless, current developmental constraints result in long generation times, but these constraints may be alleviated by the further development of structural in silico prediction.
- Hu, W. G. et al. Antibody Humanization by a Single Cycle of CDR-Grafting. Ricin Toxin. 2014; chapter 9. doi: 10.2174/9781608058785114010013
- Kurella, V. B. and Gali, R. Antibody Design and Humanization via In Silico Modeling. Methods Mol Biol. 2018; 1827:3-14. doi: 10.1007/978-1-4939-8648-4_1