Antibody production
Antibody production
Will camelid antibodies offer a solution to solve the COVID-19 pandemic?
The emergence of the zoonotic pathogen, SARS-CoV-2, resulted in a global pandemic and caused the deaths of hundreds of thousands of people. After SARS-CoV-1 and MERS-CoV, this strain is the third known to have spilled from wild animal reservoirs with severe consequences to the human population. But prior studies with these zoonotic viruses may have paved the way for the development of innovative treatments such as the use of camelid antibodies as neutralizing agents of SARS-CoV-2.
Camelid antibodies: a steppingstone in the “hunt” for the treatment of the COVID-19 disease?
Prior studies with SARS-CoV-1 and MERS-CoV have paved the way to understand the underlying mechanisms of infection in the virus responsible for the COVID-19 disease.
Only a few weeks after the identification of the new coronaviral strain, researchers were able to determine that the spike glycoprotein of SARS-CoV-2 is responsible for binding the angiotensin-converting enzyme 2 (ACE2) receptor. The binding of ACE2 catalyzes a series of complex events including proteolytic cleavage of the spike and subsequent membrane fusion.
The spike is also one of the most abundant structural proteins on the surface of SARS-CoV-2, making it an attractive target for therapeutic and prophylactic vaccine development.
As of May 2020, dozens of clinical trials are underway to test the efficiency and safety of new or repurposed antibodies for the treatment of COVID-19. However, most of these treatments focus on alleviating the symptoms of the COVID-19 disease, symptomatic treatments, instead of neutralizing the infection at its point of entry.
Previously, many antibodies have been developed against SARS-CoV-1 and MERS-CoV. These studies were successful in isolating molecules able to block the receptor-binding site and triggering the premature cleavage of the spike. By precipitating the conformation change, these molecules prevent the viral particles from interacting with the ACE2-enriched cells and stop the infection in its early stages.
Among these studies, some authors found that some anti-SARS-CoV antibodies may be capable of cross-reacting with the new strain SARS-CoV-2. Recently, a team led by Jason S. McLellan (University of Texas, US) reported the first llama-derived antibody able to neutralize the causative agent of the COVID-19 disease.
What does their discovery mean and what do we know about the advantages and limitations of using camelid antibodies for the treatment of respiratory infections?
Advantages and limitations of camelid antibodies
Camelid antibodies, first discovered in the 1990s, are heavy-chain antibodies (HCAbs) with a single variable domain (VHH).
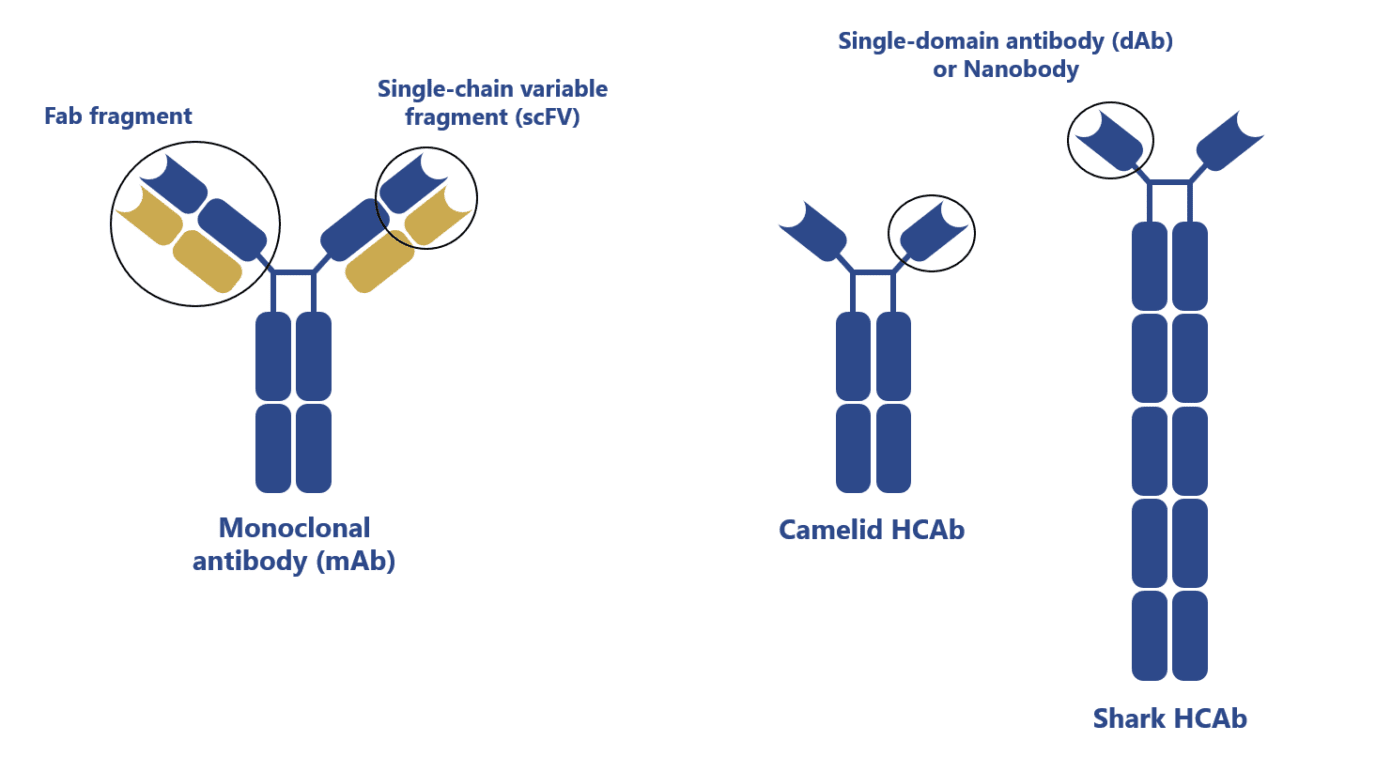
They were found to be robust, stable, and able to target unique epitopes making them highly desirable molecules for the clinic. In comparison with conventional antibodies, HCAbs or VHH (nanobodies) offer significant advantages:
- HCAbs and VHH are thermally and chemically more stable
- VHHs are more soluble (fewer aggregates) and robust than single-chain antibody fragments (scFv) derived from conventional antibodies
- VHHs can more easily diffuse across tissues than conventional antibodies
- The smaller size of VHHs makes then easier to synthesize and to use in the construction of multivalent molecules
- HCAbs and VHHs have a comparable affinity to conventional antibodies despite having only 3 and not 6 functional complementary-determining regions (CDRs)
- HCAbs and VHH show reduced susceptibility to steric hindrance
- Possibility to nebulize VHHs biologics for administration via an inhaler or at the site of infection
Despite the attractive features of these molecules, the first nanobody was only recently approved for clinical use. That antibody, Caplacizumab (Cablivi®), is a humanized VHH targeting the human von Willebrand factor and approved by the FDA in 2018 for the treatment of acquired thrombotic thrombocytopenia purpura (TTP).
Camelid antibodies are routinely obtained by screening naïve, immune, or synthetic VHH libraries using display technologies (e.g. phage display). However, some obstacles are hindering the adaptation of these molecules for the clinic. For instance:
- Despite being less immunogenic to humans than other xenogeneic antibodies, some VHH may still need to undergo humanization. Like conventional antibodies, the humanization of VHH may lead to partial or complete loss of affinity
- VHHs have limited ability to bind small molecules in comparison to conventional antibodies
- Due to its small size, VHHs exhibit a fast clearance behavior which results in a short serum half-life that needs be compensated by conjugation with PEG, Fc domains, or other molecules
What makes the new study on camelid antibodies against COVID-19 different?
The recently isolated anti-SARS-CoV-2 camelid antibody is the first VHH construct shown to block the new strain of coronavirus in vitro.
The team led by researchers of the University of Texas (US) obtained this construct by immunizing a llama with protein S from MERS-CoV and SARS-CoV-1 for 40 days. VHH sequences were recovered by RNA extraction of the peripheral blood. Using phage display, the researchers were able to select VHH specific against SARS or MERS. One of the anti-SARS-CoV-1 VHH was found to weakly cross-react with SARS-CoV-2.
In an attempt to increase the affinity, the authors of the study engineered the promising VHH to a bivalent conformation and fused it with the Fc domain of an IgG1 human antibody (VHH-72-Fc). In vitro studies with this construct, revealed that it might be able to block SARS-CoV-2 spike protein.
The cross-reactivity of this construct can be explained by the fact that, unlike previously tested anti-SARS-CoV-1 conventional antibodies, this VHH binds to a more conserved region of the receptor-binding domain. But despite the great promise of this construct, significant drawbacks may limit its application for therapeutic purposes.
For instance, these constructs may be immunogenic to humans. Moreover, the presence of a glycosylated Fc domain implies the use of mammalian expression systems, making its production a more complex and lengthy process in comparison to simple VHH production.
Concluding remarks
While a recent study proposed the use of camelid antibody constructs for the treatment of COVID-19, these antibodies still suffer from considerable drawbacks when it comes to therapeutic applications.
Nevertheless, this study shows us that llama immunization with specific SARS-CoV-2 antigens allied to phage display may be a suitable and fast technique to generate anti-SARS-CoV-2 antibodies for therapy, research, and diagnostics.
- Arbabi-Ghahroudi, M. Camelid Single-Domain Antibodies: Historical Perspective and Future Outlook. Front Immunol. 2017; 8: 1589. doi: 10.3389/fimmu.2017.01589
- Godakova, S. A. et al. Camelid VHHs Fused to Human Fc Fragments Provide Long Term Protection Against Botulinum Neurotoxin A in Mice. Toxins (Basel). 2019; 11(8): 464. doi: 10.3390/toxins11080464
- Vincke, C. et al. General Strategy to Humanize a Camelid Single-domain Antibody and Identification of a Universal Humanized Nanobody Scaffold. J Biol Chem. 2009; 284(5):3273-3284. doi: 10.1074/jbc.M806889200
- Wrapp, D. et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. 2020; S0092-8674(20)30494-3. doi: 10.1016/j.cell.2020.04.031