 Antibody production
Antibody production
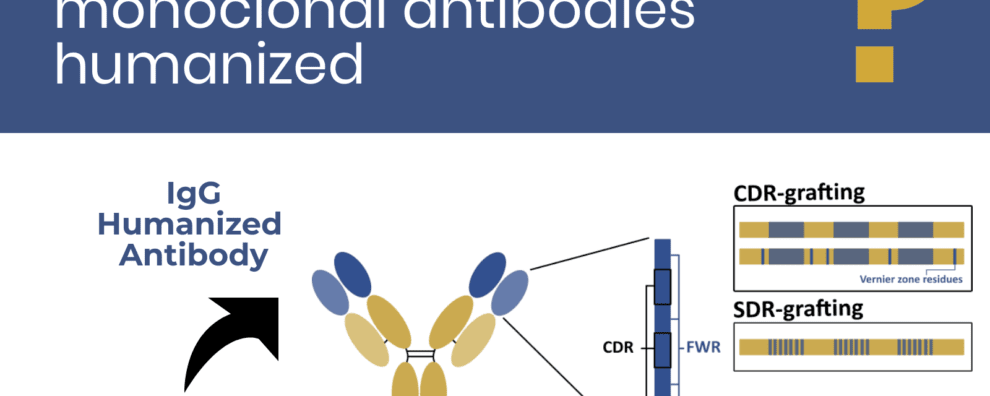
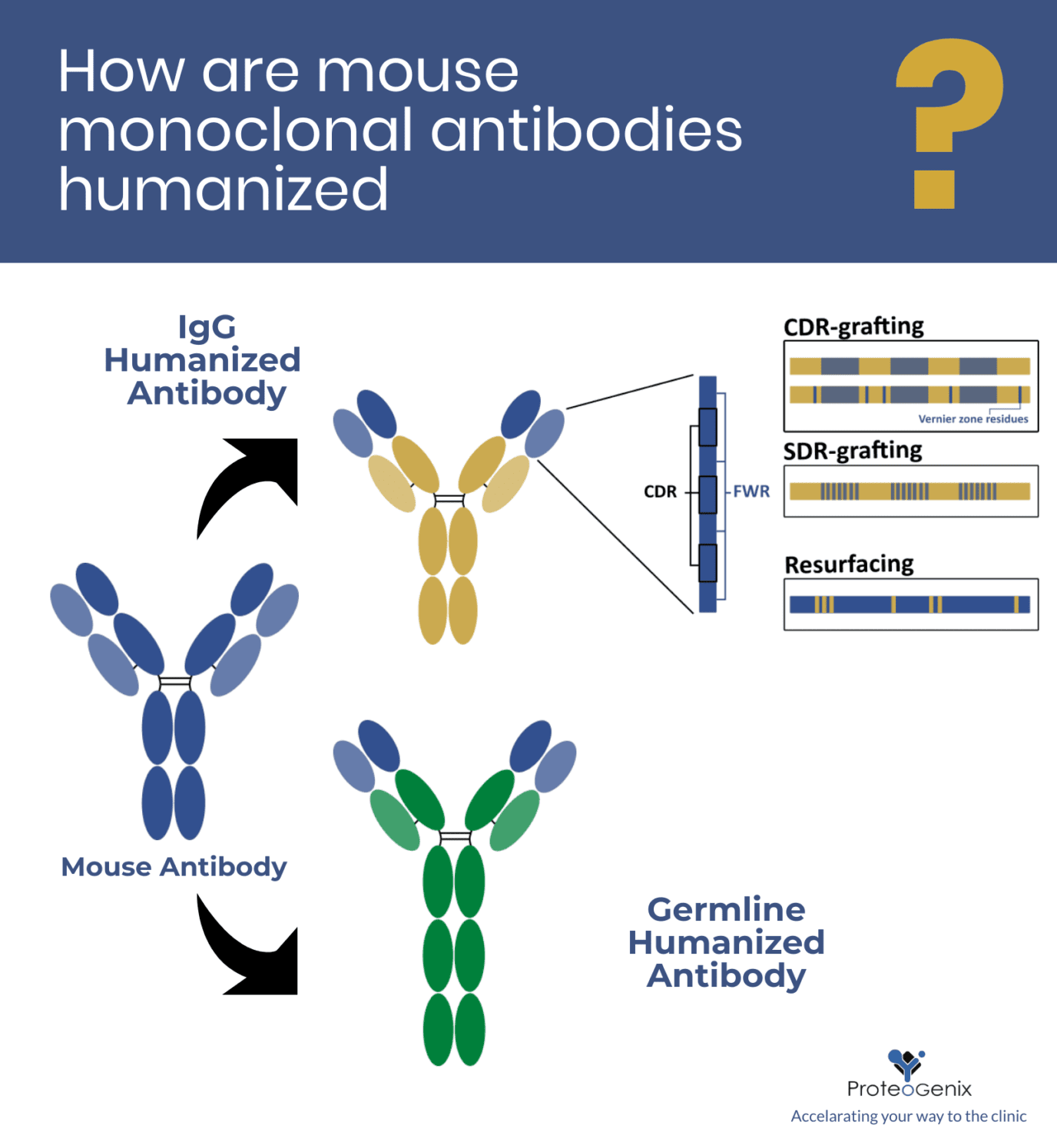
How are mouse monoclonal antibodies humanized: current approaches and limitations
Mouse monoclonal antibodies were the first to ever be produced in vitro. However, early-stage clinical evaluation of these molecules led to the quick discovery of their undesirable side-effects. These side-effects consisted of a moderate allergic response that became known as the human anti-mouse antibody (HAMA) response. Its discovery prompted the development of antibody humanization techniques that have become indispensable for the development of therapeutic antibodies from nonhuman hosts.
- Are humanized antibodies still relevant for therapeutic applications?
- Underlying principles of monoclonal antibody humanization
- Antibody IgG-based humanization
- CDR-grafting by framework region (FWR) homology
- CDR-grafting by CDR homology
- SDR-grafting
- Resurfacing approach
- Antibody germline-based humanization
- Concluding remarks
Are humanized antibodies still relevant for therapeutic applications?
As of February 2020, 82 therapeutic antibodies were granted approval by the FDA or EMA for clinical use in the US or EU, respectively. Of these, 39 are humanized immunoglobulins generated in mice or rabbits. This data reflects the fruits of the past decade of research in antibody humanization. Moreover, it shows that humanized antibodies still stand on equal terms with fully human antibodies when it comes to rates of clinical approval.
Over the years, scientists developed different techniques to accomplish antibody humanization. Interestingly, many of these techniques seem to apply to antibodies independently of their original host. For this reason, as antibodies from species like rabbit and camelids become increasingly relevant, so will the use of techniques that aim at humanizing these biomolecules.
Underlying principles of monoclonal antibody humanization
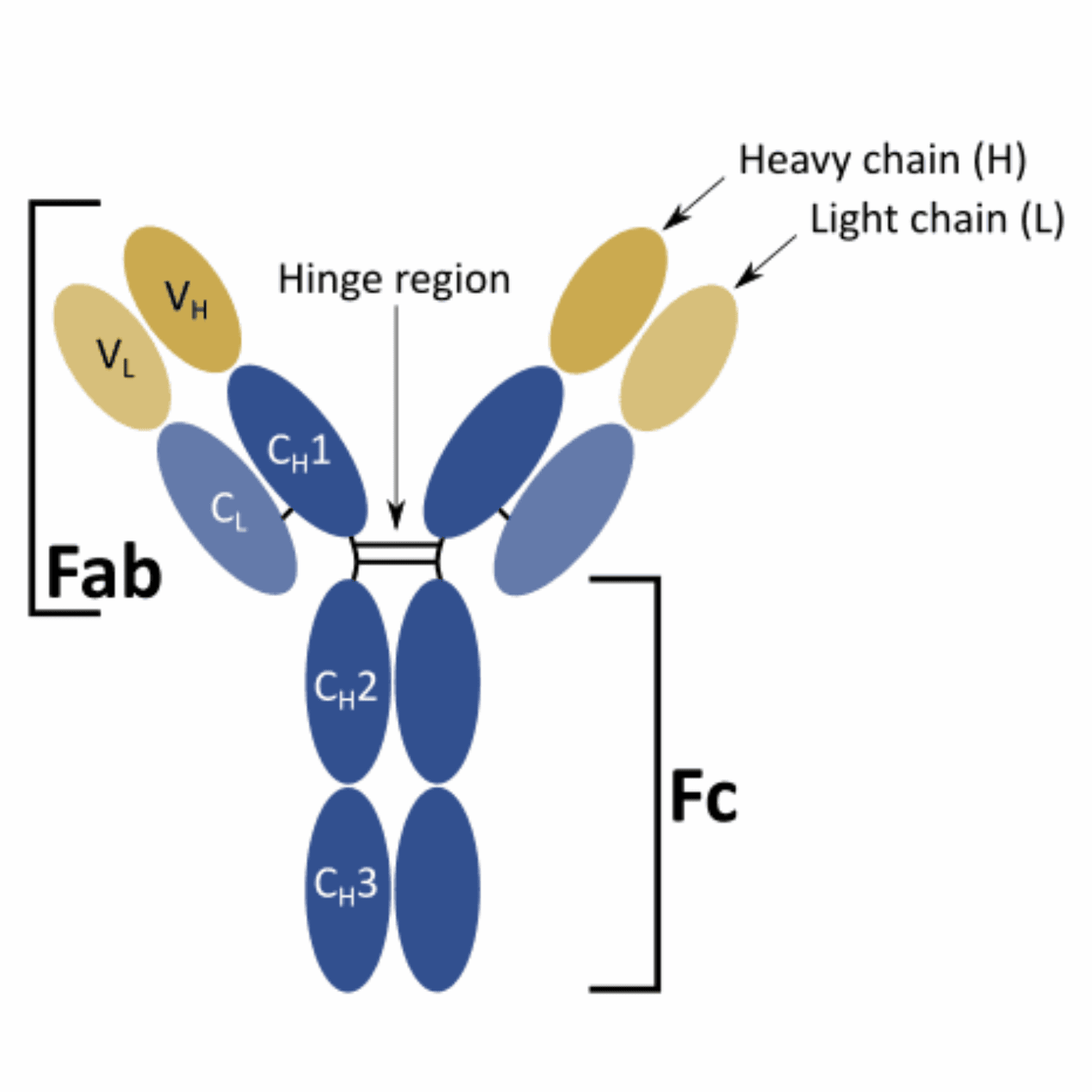
Immunoglobulins consist of four peptide chains – two identical heavy chains (H) and two identical light chains (L). Each chain contains a variable (V) and a constant domain (C), responsible for interacting with the antigen or with other key elements of the immune system, respectively. Within each V domain (VL and VH) there are three non-consecutive complementarity-determining regions (CDRs) loops or hypervariable regions, that directly interact with an antigen.
For this reason, CDRs and adjoint regions contain the most important information regarding antigen-specificity in a given molecule. This knowledge has led to the development of one of the most popular methods of antibody humanization – CDR-grafting – which takes these regions and integrates them into the framework of a human antibody. Thus, reducing the risk of the molecule being recognized as foreign by the patients’ immune system.
Most antibody humanization techniques start by generating a chimeric IgG-based antibody containing the V region of the xenogeneic molecule and the C region of a typical human IgG molecule. After the expression of a stable chimeric molecule, humanization efforts focus on the V region itself through different approaches.
Antibody IgG-based humanization
CDR-grafting by framework region (FWR) homology
To this day, CDR-grafting continues to be the preferred method for antibody humanization. This technique implies the use of a “donor” antibody containing the antigen-specific CDRs and the “acceptor” antibody that will serve as the framework for the hybrid molecule. The key step of this technique is thus the choice of an adequate “acceptor” molecule.
Typically, human frameworks with the highest homology to the same regions of the xenogeneic antibody are chosen as “acceptor” molecules.
Interestingly, early attempts at humanizing mouse antibodies through the CDR-grafting approach resulted in a significant loss of affinity and specificity. In many cases, this loss resulted from inaccurate annotation of the CDRs sequences, inappropriate choice of human frameworks, and erroneous identification of homologous regions between the “donor” and “acceptor” molecule.
However, it soon became evident that grafting the CDR residues alone was insufficient to guarantee the binding efficiency, because adjacent residues could also affect the conformation of the antigen-binding site. These key regions in the vicinity of the CDR loops became known as “Vernier zone residues”. They were shown to affect the stability, flexibility, and structure of the CDR loop, and thus, impact the affinity and specificity of the molecule. For this reason, current CDR-grafting strategies now include the grafting of these complementary regions as well.
CDR-grafting by CDR homology
Interestingly, other authors have taken a completely different approach on how to match “acceptor” and “donor” antibodies. Instead of focusing in the homology between the framework regions (FWR) of the human and xenogeneic molecule, some researchers are focusing on matching these molecules based on the homology of the CDR regions. Why? Because of the importance of adjacent residues to the conformation of an antibody.
Unlike the conventional CDR-grafting approach, humanization by CDR homology does not attempt to preserve the residues from the Vernier zone, instead, it tries to find a pre-existing human IgG antibody with comparable structural organization. Antibodies generated by this method were found to retain a significantly higher affinity than antibodies generated via the framework region homology method.
These results suggest this method is a suitable alternative to the current approach at CDR-grafting humanization. However, to the best of our knowledge this approach has not become as widespread as the former in therapeutic antibody development. Possibly because CDR-grafting by FWR homology was developed earlier than this alternative approach.
SDR-grafting
Antibodies humanized by the CDR-grafting technique may still elicit an immune response in patient. To minimize this response, the grafted regions may be further restricted to the specificity-determining residues (SDRs).
SDRs are the most important residues involved in antibody-antigen interaction within the CDRs. By grafting these residues alone, instead of grafting the entire CDR, researchers aim at reducing the anti-idiotypic (anti-Id) response. Several researchers have tested this approach in murine antibodies and concluded that these molecules had a binding affinity comparable to their CDR-grafted counterparts and showed a lower immunogenicity in patients.
SDR-grafting follows the same principles as conventional CDR-grafting by FWR homology in terms of choice of appropriate human frameworks and correct annotation of the CDR region. However, little work has been published recently on this topic, suggesting that SDR-grafting is not a widely used technique for antibody humanization.
Resurfacing approach
The variable domain resurfacing approach to antibody humanization emerged in the early 1990s. Unlike SDR and CDR-grafting, the resurfacing method takes a more holistic approach to antibody humanization.
Instead of focusing on specific and well-defined regions, resurfacing seeks to identify surface framework residues that can potentially cause an immune response in patients, and replace them by the most common residues found in human antibodies in those same positions. In other words, resurfacing implies the replacement of problematic residues in the FWR surface of the xenogeneic molecule, by safer residues typically found in human antibodies.
Several studies show that antibodies humanized by this approach had a longer half-life, reduced immunogenicity, and comparable in vitro activity. However, very few antibodies have actually been humanized using this approach, suggesting there is a need to gather more data to understand the real potential to this approach at humanization.
Antibody germline-based humanization
Of all the current approaches to antibody humanization, germline-based humanization is perhaps the most innovative and controversial one. It is based on the knowledge that human germline frameworks are better tolerated by the immune system than framework sequences from fully mature IgG antibodies. Unlike germline sequences (pre-affinity maturation), the framework of IgG antibodies undergoes many somatic hypermutations during the affinity maturation process. For this reason, even fully human IgG molecules are known to cause an allergic reaction, known as the human anti-human antibody (HAHA) response.
Despite some antibodies have been humanized through this approach and still managed to retain their therapeutic effectiveness, there also has been some studies reporting the opposite effect. For this reason, more research is necessary to understand the true potential of this approach.
Concluding remarks
Antibody humanization attempts to reduce the immunogenicity of xenogeneic-derived antibodies. First developed used mouse monoclonal antibodies as frameworks, humanization can now be applied to antibodies generated across many different hosts.
To this date, humanization by CDR-grafting and FWR homology continues to be the most prevalent approach. However, many studies revealed that it is increasingly important considering the entire three-dimensional structure of an antibody before devising a humanization approach.
For this reason, humanization will likely evolve to include many more modelling and in silico studies to reduce the risk of loss of affinity.
- Ahmadzadeh, V. et al. Antibody humanization methods for development of therapeutic applications. Monoclon Antib Immunodiagn Immunother. 2014; 33(2):67-73. doi: 10.1089/mab.2013.0080
- Dondelinger, M. et al. Understanding the Significance and Implications of Antibody Numbering and Antigen-Binding Surface/Residue Definition. Front Immunol. 2018; 9: 2278. doi: 10.3389/fimmu.2018.02278
- Kashmiri, S. V. et al. SDR grafting–a new approach to antibody humanization. Methods. 2005; 36(1):25-34. doi: 10.1016/j.ymeth.2005.01.003
- Safdari, Y. et al. Antibody humanization methods – a review and update. Biotechnol Genet Eng Rev. 2013; 29:175-186. doi: 10.1080/02648725.2013.801235

