Cart (0 Items)
Your cart is currently empty.
View Products
Client
Small French biotech company
Sector
Therapeutic
Research Domain
Oncology
Timeline
3 weeks
Key processes
A small French biotech company was developing a mouse-derived antibody for human therapeutic applications in cervical cancer. They partnered with us to support their antibody humanization program and accelerate their path to market.
Our objective was to adapt the antibody to facilitate its transition toward clinical development by converting its sequences toward human germline frameworks. At the same time, we improved its original antigen-binding properties, maintaining specificity and performance.
Preserving antigen recognition despite framework substitutions
Preserving native VH–VL pairing compatibility
Preventing framework-driven CDR conformational shifts
For this project, we adapted our workflow to the client’s format and target requirements. Our process progressively transforms the original antibody toward the target species’ germline sequences using our proprietary AI tool.
AI-driven germline selection
The client provided the parental antibody sequence, and we targeted 3 candidate germlines in the desired species with the highest predicted structural homology.
Mutation design & risk stratification
We used our proprietary AI-guided platform to design a stepwise series of mutations, generating 22 humanized variants.
Structural validation
To control structural integrity, we compared each variant to the parental antibody using VH/VL quaternary structure, CDR conformational fluctuation, and framework structural deviation.
Expression & experimental validation
We expressed the 22 variants using our high-yield antibody expression system XtenCHO Race™, and we validated activity using ELISA to confirm they retained antigen binding.
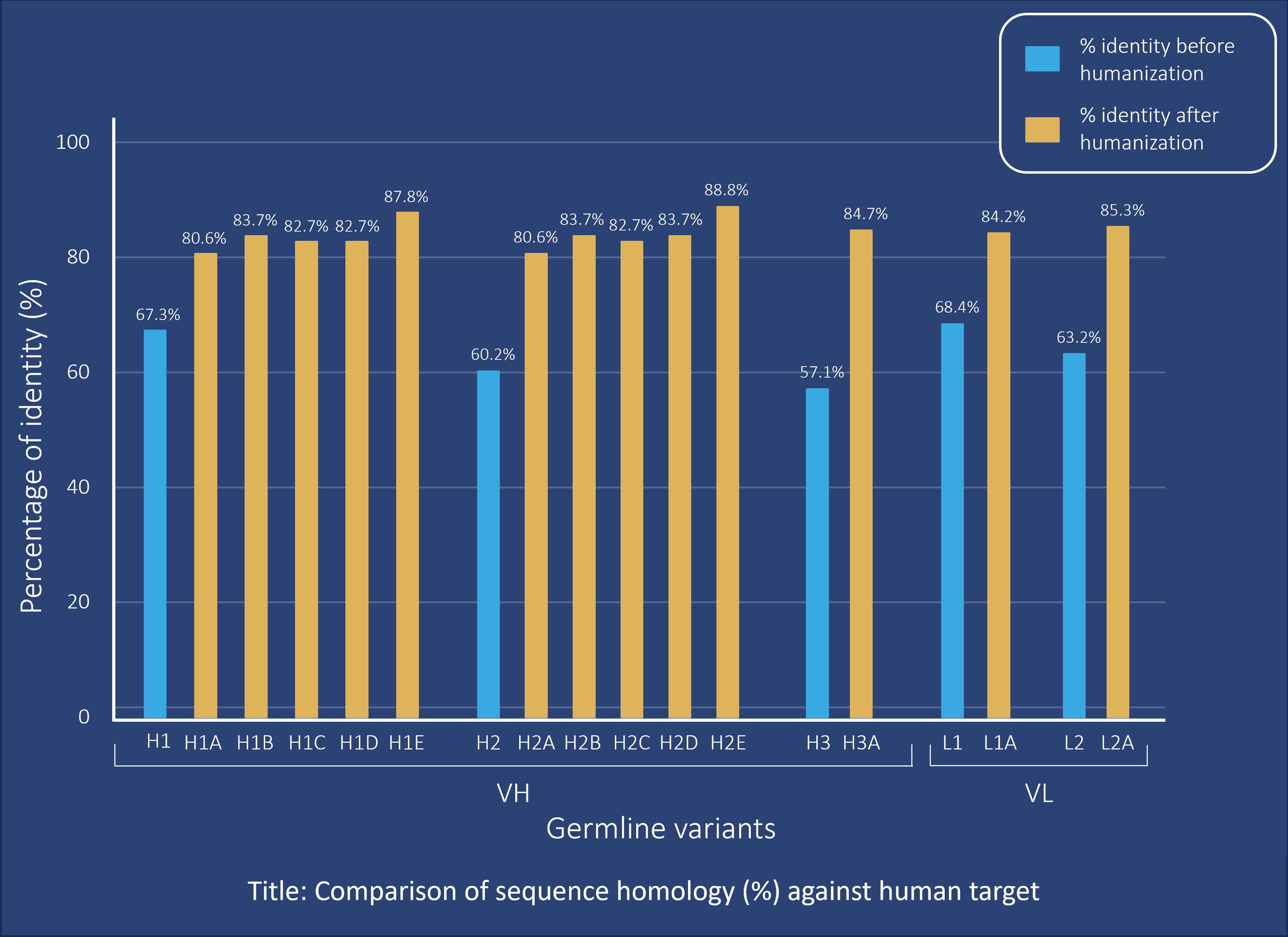
Sequence analysis confirmed that the adapted variants were consistently classified as human by germline assignment tools (see figure), therefore strongly reducing the immunogenicity risk.
Our validation structure step showed the preservation of the overall VH/VL architecture compatible with antigen recognition and the dynamic architecture of the paratope. In addition, ELISA results showed that several variants achieved up to a 2.6-fold increase in apparent binding compared with the parental antibody. Based on these data, we prioritized three lead candidates for further characterization: VHVL05, VHVL10, and VHVL22.
As part of the full project delivery, we provided a detailed report with tests results, the 3 purified top variant samples and the complete set of adapted sequences across all evaluated VH/VL combinations. We delivered additional sequence variants while preserving the antibody’s key attributes to maximize the chances of success. Detailed sequence alignments ensured full transparency, enabling the client to quickly validate and select the most suitable candidate.
This extensive panel of humanized antibodies, including 3 lead candidates, gave the client a broad range of options to select the optimal variant and ultimately strengthen the therapeutic development strategy.
Our fast, AI-based antibody species adaptation process minimized immunogenicity risk (>80% homology to the human sequence) while also increasing antigen binding (up to 2.6-fold increase).