Cart (0 Items)
Your cart is currently empty.
View Products


| size | 100µg |
|---|---|
| Isotype | IgG1 |
| Brand | ProteoGenix |
| Product type | COVID-19 products |
| Clonality | Monoclonal Antibody |
| Product name | Anti-RBD-5 antibody (Imdevimab) |
|---|---|
| Species | Human |
| Expression system | Mammalian |
| Molecular weight | 150kDa |
| Purity | 85% |
| Buffer | PBS, pH7,5 |
| Form | Liquid |
| Delivery condition | Blue ice (+4°C) |
| Storage condition | 4°C for short term; -20°c or -80°C for long term |
| Brand | ProteoGenix |
| Host species | Mouse |
| Aliases /Synonyms | REGN10987 |
| Reference | PTXCOV-A553 |
| Note | For research use only. Not suitable for human use. |
| Isotype | IgG1 |
| Clonality | Monoclonal Antibody |
| Target | RBD domain- SARS-CoV2 Spike protein for first type and variants (PX-COV-P046, PX-COV-P052, PX-COV-P053, PX-COV-P054, PX-COV-P056) |
| Target species | SARS-CoV-2 |
The anti-CoV-RBD antibody is an anti-SARS-CoV-2 antibody with potential neutralizing activity. By screening its receptor-binding domain (RBD), extensive library of COVID-19 antibodies (LiAb-SFCOVID-19 *) identify the new coronavirus. Variants produced in IgG format have shown stable stability when expressed in XtenCHO ™ (transient expression system based on the created cell line) and consistently provide high and consistent amounts.
In an ELISA (enzyme-linked immunosorbent assay) plate, the specificity and affinity of the IgG antibody were tested against the purified and recombinant form of RBD. Furthermore, the SARS-CoV-2 alternative virus neutralization test (sVNT) indirectly confirmed the neutralization ability. This test is designed to quickly and accurately detect the ability of antibody variants to block the interaction between RBD and specific human receptors. ACE2.
Since the beginning of the pandemic, many studies have confirmed the therapeutic potential of SARS-CoV-2 NAb (neutralizing antibody). The reason RBD takes precedence over other SARS-CoV-2 domains is its key role in the emergence of viral infections. By preventing this important interaction, the researchers hope to halt the development of the disease. Antibody therapy also shows unique advantages over small molecules in the treatment of COVID-19. In fact, most antiviral medications suppress the immune response, which generally delays the clearance of virus particles from the patient. In contrast, antibody therapy further engages and activates the individual’s immune system, resulting in a synergy between the blocking activity of the antibodies and a faster and safer removal of virus pathogens. In this case, the investigation of neutralizing antibodies (such as anti-CoV-RBD (E4)) can help to investigate the progression of the disease and the response of the body to specific therapies.

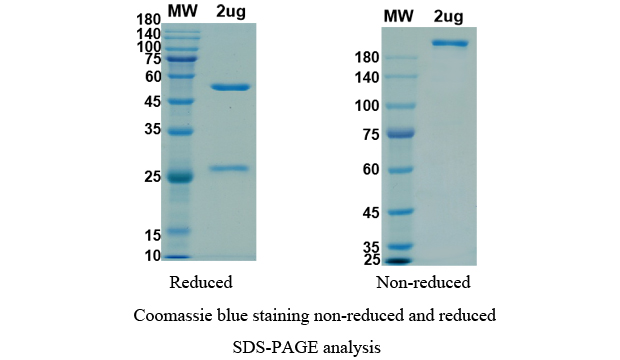
Anti-RBD-5 antibody (Imdevimab), on SDS-PAGE under reducing and non-reducing conditions. The gel was stained overnight with Coomassie Blue. The purity of the protein is greater than 95%.
Related products
Send us a message from the form below
Toshi –
This antibody along with other therapeutic antibodies were tested in surrogate virus neutralization test using spike trimers and ACE2 protein. This antibody showed expected neutralization of wild type, Alpha, Gamma, and Delta variants (IC50 5.28 ng/mL for the wild type, 4.6 ng/mL for Alpha, 4.76 ng/mL for Gamma, 3.86 ng/mL for Delta).