Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | Human IgG |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Sotrovimab Biosimilar - Anti-Spike glycoprotein mAb - Research Grade |
|---|---|
| Species | Humanized |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery lead time in business days | 3-5 days if in stock; 3-5 weeks if production needed |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Sotrovimab ,,Spike glycoprotein,anti-Spike glycoprotein |
| Reference | PX-TA1637 |
| Note | For research use only. Not suitable for human use. |
| Isotype | IgG |
| Clonality | Monoclonal Antibody |
Sotrovimab is a neutralizing monoclonal antibody with an activity against SARS-CoV-2 virus in humans. It has been commercialized as XEVUDY by GlaxoSmithKline and Vir Biotechnology, Inc. Sotrovimab is able to bind with Spike protein, which is responsible of the pathogenicity of the Severe Acute Respiratory Syndrome- Coronavirus( SARS-CoV-2).
FDA and EMA have very recently given their approvals for the marketing of Sotrovimab as a treatment against some severe forms of COVID. The epitope targeted by this neutralizing antibody is not mutated in the different variants of SARS-CoV-2 of concern.
Spike glycoprotein is a transmembrane viral fusion protein. It is known to be paramount in the binding of the virus to the cell during infection process. Spike has a homotrimeric structure and each monomere is composed of two subunits: S1 and S2. The S1 part is carrying the Receptor Binding Domain (RBD), and the S2 part is responsible of the fusion of the virus with the cellular membrane. The RBD Part of S1 is binding the Angiotensin Converting Enzyme 2 (ACE2) in the organism.
Sotrovimab biosimilar is a dual-action antibody, which is able to to neutralize the virus in vitro, kill infected cells, provide a high barrier to resistance, and achieve high concentrations in the lungs. Proteogenix offers this product for research use only. Sotrovimab is a human-origin monoclonal antibody expressed in a mammalian system (Chinese Hamster Ovaries-CHO).

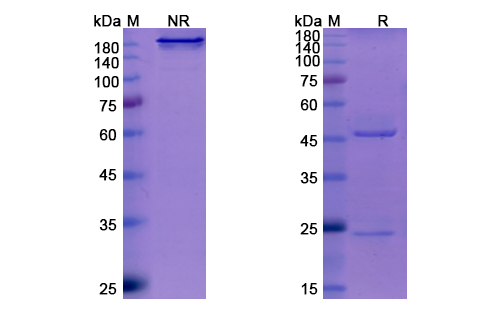
Sotrovimab Biosimilar - Anti-Spike glycoprotein mAb, on SDS-PAGE under reducing and non-reducing conditions. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.

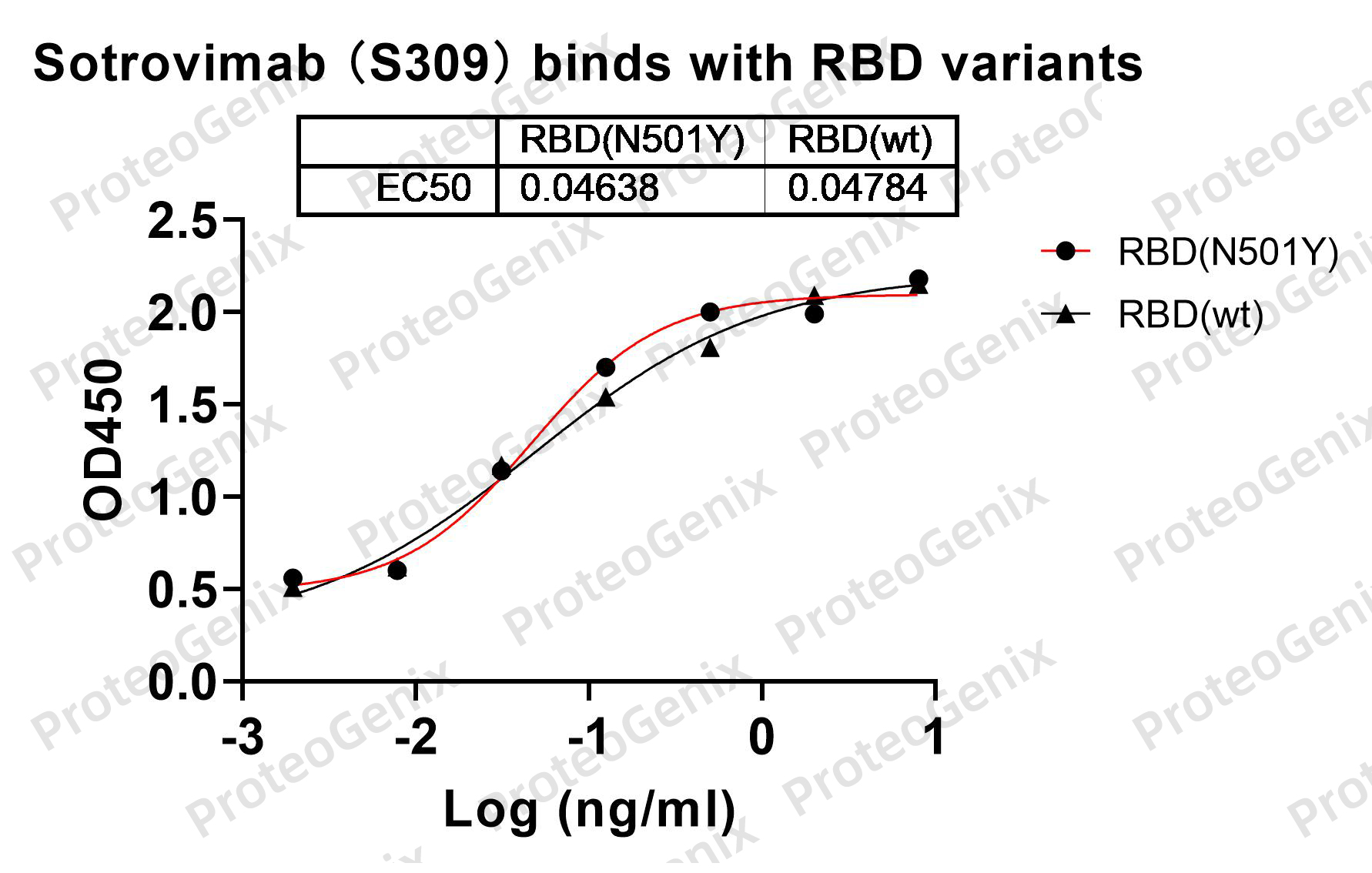
Immobilized RBD Domain (cat. No.PX-COV-P046) at 0.5µg/mL (100µL/well) can bind to Sotrovimab Biosimilar - Anti-Spike glycoprotein mAb (cat. No.PX-TA1637) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Send us a message from the form below
Toshi –
This antibody along with other therapeutic antibodies were tested in surrogate virus neutralization test using spike trimers and ACE2 protein. This antibody did not show weak inhibition of all variants tested (IC50 344.03 ng/mL for Delta, >500 ng/mL for Gamma and Alpha, 244.1 ng/mL for the wild type).