Cart (0 Items)
Your cart is currently empty.
View Products
Multidrug-resistant bacteria cause the deaths of an estimated 1.4 million people annually (1). To combat antibiotic-resistant superbugs and prevent a projected 10 million deaths by 2050, Australia-based Recce Pharmaceuticals has engineered an entirely new class of anti-infectives — a feat that hasn’t been achieved in almost 40 years.
Recce’s lead candidate, RECCE® 327, is a fast-acting, broad-spectrum antibiotic polymer that overwhelms bacteria by simultaneously interfering with numerous bacterial pathways. The result is potent bactericidal properties.
However, advancing the clinical development of RECCE® 327 requires the ability to detect and quantify the drug in vivo. This necessitates the creation of a custom monoclonal antibody that can detect the compound in complex matrices with precision and support existing in-house methods.
But there was a problem. Such an antibody not only didn’t exist — other monoclonal antibody development suppliers had not succeeded.
Recce Pharmaceuticals Chief Scientific Officer and Co-inventor, Michele Dilizia, explains that monoclonal antibody suppliers “[were] unsuccessful because they centred on traditional approaches and conventional methods.”
We sat down with Ms. Dilizia in an exclusive interview where she reveals “the qualities that allowed ProteoGenix to succeed where other suppliers failed…” Understanding these challenges requires a deeper look at Recce Pharmaceuticals and its drug pipeline.
Founded in Australia, Recce Pharmaceuticals Ltd (ASX:RCE, FSE:R9Q) is a biotechnology leader developing a new class of synthetic polymer-based anti-infectives to combat antibiotic-resistant superbugs.
The clinical potential of their drug pipeline isn’t going unnoticed. Global health and regulatory authorities have acknowledged RECCE® 327 as a promising candidate against critical bacterial threats, with accelerated approval pathways granted for RECCE® 327 to treat severe infections. In Australia, regulators have also authorized its limited use by healthcare providers under specific conditions.
This innovative class of anti-infective synthetic polymers targets bacterial membranes and disrupts essential cellular processes, rapidly eliminating bacteria in stationary and growth phases. By attacking multiple sites simultaneously, these compounds significantly reduce the likelihood of drug resistance, offering a powerful approach to combating superbugs.
However, developing a unique monoclonal antibody to detect RECCE® 327, a complex anti-infective consisting of hundreds of various and repeating copolymer compounds was a barrier that Recce Pharmaceuticals historically struggled to overcome. Here is why.

RECCE® 327 is “a complex synthetic polymer comprising a mixture of some hundreds of various copolymer compounds,” explains Ms. Dilizia. What’s more, the synthetic polymer is within “a matrix that has similar chemical groups and moieties.”
Therefore, custom antibodies needed to recognize unique epitopes on RECCE® 327 without binding to a common chemical element shared across the polymer’s matrix—a challenge complicated by the molecule’s size and chemical similarity to its surroundings.
Ms. Dilizia recalls, “The approach and its methodologies [from former suppliers] were not suitable for providing antibodies for our test article.”
“We needed custom monoclonal antibody services that could deliver highly specific antibodies and tailored assays,” Ms. Dilizia recalls.
This set the stage for ProteoGenix to overcome this critical roadblock.
Recce chose ProteoGenix for their proven expertise in complex antibody projects. “The qualities that allowed ProteoGenix to succeed where other suppliers may fail were preparedness and scientific expertise to approach the project using customised methods,” Ms. Dilizia recalls. Unlike suppliers offering generic workflows, ProteoGenix provided a tailored strategy to address the unique challenges of RECCE® 327.
This partnership made ProteoGenix an extension of Recce’s team, aligning with their scientific and regulatory goals.
To meet Recce’s challenge, ProteoGenix deployed a sophisticated hybridoma-based strategy. “Developing antibodies that only recognized RECCE® 327’s unique epitopes required extreme specificity,” Ms. Dilizia notes. The complexity of the small-molecule polymer, coupled with a common chemical element in its matrix, demanded innovative methods.
ProteoGenix developed an innovative solution. They created a hapten by conjugating RECCE® 327 to a protein, creating an immunogenic antigen. This was a critical step given the small molecule’s limited epitope size.
ProteoGenix’s custom monoclonal antibody development solution, backed by scientific expertise, was pivotal to overcoming the challenge.
The project unfolded through critical milestones, each advancing Recce’s goal of precise pharmacokinetic monitoring in vivo:
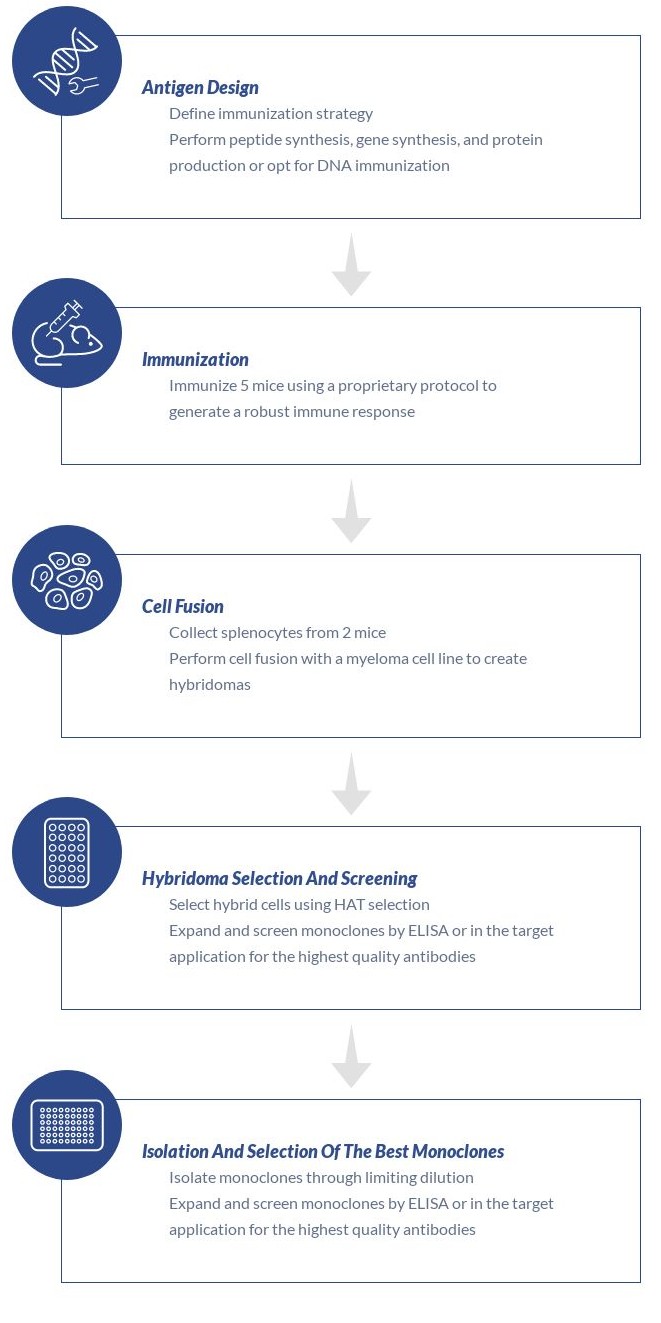
ProteoGenix delivered six murine IgG hybridomas with exceptional specificity for RECCE® 327, detecting the antibacterial compound without cross-reacting with its common chemical element. These antibodies have enabled Recce to develop robust pharmacokinetic assays, important for RECCE® 327 drug development and regulatory approval.
The delivered monoclonal antibodies supported:
“An indication of [the delivered antibodies] great value can be obtained when considering the important role of antibodies to assess a drug’s pharmacokinetic parameters – eg, Absorption, Distribution, Metabolism and Excretion,” Ms. Dilizia says. “This ADME profile is sought by global regulatory agencies and is an important component of successful drug development.” Thus, overcoming this pharmacokinetic hurdle helped RECCE® 327 get one step closer to success.
“The key benefit of partnering with ProteoGenix was being able to hold scientific discussions with their expert team to explore the overall approach and methods, versus the ‘one size fits all’ approach offered by other providers”
“ProteoGenix was willing to apply effort and critical thinking to deliver a very successful outcome.”
The partnership’s lasting value includes:
“I would highly recommend the services of ProteoGenix to other researchers.” — Ms. Dilizia concludes, citing their scientific depth, customization, and communication.
With the successful development of 3 antibodies and 30+ more in clinical and preclinical trials, Recce Pharmaceuticals is just one of our many success stories. We want you to be our next success. With over 25 years of antibody engineering experience, strict go-no-go checkpoints to safeguard your investment, and a 90 %+ success rate, it’s easy to see why pharmaceutical companies and academic institutions alike trust ProteoGenix to be an extension of their team.
Book a free call today and discover why Recce Pharmaceuticals and thousands of researchers trust ProteoGenix’s monoclonal antibody development services.
1. GBD 2021 Antimicrobial Resistance Collaborators. (2024). Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. The Lancet, 404(10459), 1199–1226. https://doi.org/10.1016/S0140-6736(24)01867-1