Cart (0 Items)
Your cart is currently empty.
View Products
Streamline CAR-T cell development thanks to our CAR-T cell platform. Ally the high diversity of our antibody libraries (> 1010) with our vast experience in therapeutic antibody development (3 biopharmaceuticals on the market and 30 undergoing clinical trials) to build highly specific CARs for your applications. Starting at 12 weeks.
Achieve optimal affinity thanks to our diversified antibody discovery solutions: hybridoma or single B-cell with multiple immunization strategies (protein, peptide, DNA, small molecule) or phage display of immune or naive libraries (> 1010 clones).
Get full ownership of the CAR sequences generated!
Forgo the need for antibody humanization and simplify CAR development thanks to our high diversity human library LiAb-SFMAXTM with over 1010 clones.
Starting from any species including human.
Get your CAR construct in as little as 12 weeks.
Drawing from 20+ years of experience in therapeutic antibody development allows us to propose the best solutions adapted to your unique needs.
In this video, Karim Sebane, Business Manager for Monoclonal Antibodies at ProteoGenix, takes us inside a particularly complex and rewarding CAR-T project he helped bring to life.
From stabilizing a GPCR in its native conformation to discovering fully human antibodies and designing the final CAR construct, Karim walks us through the key choices and strategies that made this ambitious program a success.

Chimeric antigen receptors (CARs) are synthetic structures designed to modulate natural T cell receptors (TCRs). They consist of four main components:
CAR structures differ from TCR in the way they interact with the antigen. TCRs only bind peptides that are bound and presented by major histocompatibility molecules (MHC). In contrast, CARs bind antigens that are directly bound to the membrane of cancer cells.
This small difference in terms of structure and recognition has a huge impact in terms of function. MHC loss or downregulation is a common mechanism employed by cancer cells to evade the organism’s immune response. For this reason, most cancer antigens are displayed on the surface of the cell membrane not bound by MHCs. By forgoing MHC-mediated antigen recognition, CARs become invaluable for treating immune escape refractory cancers (that do not respond to any other treatment).
To elicit a specific anti-cancer response, CARs are introduced into T cells where they will redirect their cytotoxic activity towards tumor cells.
New perspectives for CAR-T therapy
Explore how we designed antibodies against a target
barely visible to the immune system
The first T cells engineered to express CARs were developed in the late 80s and early 90s. Reports of an effective application of this immunotherapy was published as early as 2010. Only seven years later, the first of these “living drugs” received marketing approval by the Food and Drug Administration (FDA).
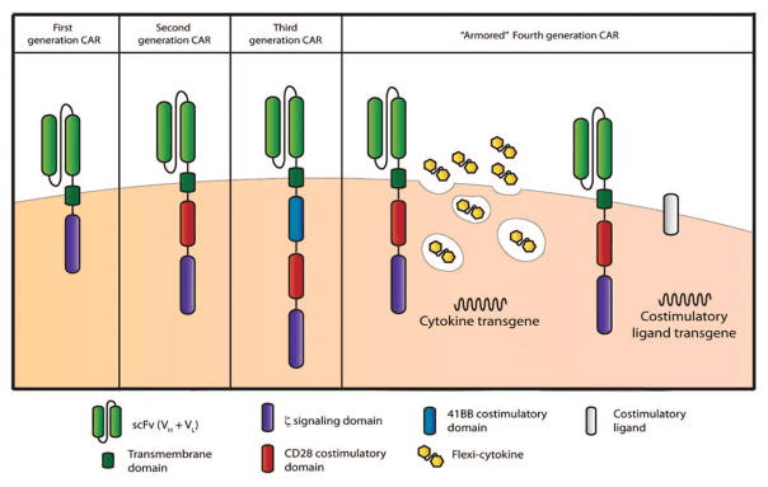
Despite their recency, the structure of CARs has evolved tremendously since their inception. From the first to the latest generation of CARs, these constructs have kept their canonical structure (extracellular, transmembrane, and intracellular) but evolved significantly in terms of composition and additional domains to increase signal strength and sustain the cytotoxic response.
To date, four generations of CAR-T cells have been described and a fifth in underway. In each new generation of CARs, the signaling domains are modified while the other domains remain constant:
CAR-T cells are a form of adoptive T cell therapy (ACT), a type of immunotherapy that relies on the therapeutic use of T cells. Due to their complex intracellular domains, CAR-T cells are known to leverage different mechanisms to eliminate tumor cells. These mechanisms are triggered in response to a specific tumor-associated antigen. Once the antigen-binding domain (scFv, sdA, or VHH) binds the antigen, an immune synapse forms in the interface between the two cell types.
Lysis subsequently occurs via three known pathways:
The process of manufacturing CAR-T cells requires two components: a well-developed CAR and a source of T cells. All CAR-T cell therapies approved to date are manufactured by sourcing T cells from the patients who will receive the treatment – also named autologous T cells. These cells are harvested by leukapheresis, a form of apheresis designed to extract lymphocytes from the blood of patients. The system does so by centrifugation or membrane filtration followed by reinfusion of the remaining components together with replacement fluid.
These T cells are subsequently activated using antibodies or interleukins. Activated cells are subsequently modified by delivery via transduction (viral vectors) or transfection (plasmids) of CAR components. CAR-positive cells are subsequently expanded in vitro and reinfused back into the patient.
CAR-T cells are currently considered a last-line treatment reserved to patients with refractory tumors, unresponsive to conventional chemotherapy or immunotherapy. Most patients eligible for CAR-T cell therapies have previously received aggressive immune-depleting treatments. For this reason, the development of cell therapies based on allogeneic T cells (sourced from healthy individuals) remains one of the most important trends in CAR-T cell manufacturing. Allogeneic cell therapies alongside the construction of cell banks may propel these therapies to the first-line of anti-cancer treatments.
CAR-T cell therapies have demonstrated their effectiveness across multiple clinical trials. However, from a technical maturity viewpoint, the technology is still considered new. For this reason, many technical challenges remain to be solved until its widespread use becomes possible. Most challenges are tied to unspecific toxicity, limited activity, difficulties in penetrating solid tumors and in targeting antigen escapes.
Much effort has been put into CAR-T cell engineering in an attempt to solve these limitations. In the table below, you can find a summary of the most promising approaches.
| Challenge | Antigen escape and off-target toxicity | Limited effectiveness against solid tumors | Systemic toxicity |
|---|---|---|---|
| Solutions | Dual targeting CAR-T cells | Combinatorial therapies | Controling CAR-T cell activation in vivo |
| Description and advantages | When CAR-T cells recognize more than one antigen (antigen A or B), the chances of antigen escape leading to treatment failure are minimized
When CAR-T cells are required to bind two antigens at the same time to trigger the cytotoxic response (antigens A and B), the risks of off-target toxicity are also minimized |
The tumor’s extracellular matrix works as a physical barrier blocking access to treatments. When CAR-T cells co-express matrix-degrading enzymes, it improves tumor penetration
Solid tumors are notorious for their strong immunosupressive environments. In this environment, immune checkpoint inhibitors, were shown to enhance the effectiveness of CAR-T cell therapies |
Many strategies have been developed and successfully used to reduce the systemic toxicity of this therapy:
• Reduce CAR affinity when targeting abundant and highly expressed antigens • Use human or humanized antigen-binding domains to reduce immunogenicity • Insert molecular switches into CAR constructs allowing shut down of the response and the onset of adverse reactions |
NANOBODY® compound is a registered trademark of Ablynx N.V.
