Cart (0 Items)
Your cart is currently empty.
View Products
You struggle to access to your hidden target epitope? With our VHH screening service, get the maximum number of VHH binders with the highest binding affinity, thanks to our wide VHH antibody phage display libraries (1.51×1010 different clones). Choose your antigen of interest (peptide, protein, cell…) and we make sure you receive at least 3 highly specific binders within maximum 7 weeks.
We guarantee at least
3 binders to your
antigen of interest.
Get your full VHH
antibody sequence within
maximum 7 weeks.
Be the owner of the VHH
antibody sequence that we
generate for you.
Maximize the number of relevant
binders using our high diversity
VHH library with 1.51×1010
different clones.
Our library is tested against a
wide variety of antigens: proteins,
peptides, small molecules, whole
cells…
Benefit from our over 20
years custom monoclonal antibody development and
phage display expertise.
In Silico epitope mapping is a fully software-based technique used to computationally predict the binding site of the
antibody of interest with its antigen, or for preliminary epitope binding investigation.
Antigen design
VHH library screening and Biopanning
Screening and validation by ELISA
Phage DNA extraction + antibody screening
VHH sequence delivery to the customer
We were asked by a customer to identify at least 10 different binder sequences against an antigen (recombinant protein) that he provided us. Biopanning was performed using our VHH camelid naive library of a very high diversity. A total of 30 positive clones was screened and 14 different unique sequences with strong and specific binding were delivered to the customer.
| Rounds | Antigen concentration Dae | Number Of Washings | Phage Quantity(pfu) | Phage Quantity(pfu) |
|---|---|---|---|---|
| Phage Quantity(pfu) | Input | Phage Quantity(pfu) | ||
| 1 | 50ug/ml | 5 | 2.0*1010 | 2.5*106 |
| 2 | 50ug/ml | 5 | 2.0*1010 | 5.2*108 |
| 3 | 50ug/ml | 6 | 5.0*1011 | 6.0*108 |
Data show significant enrichment over the rounds (bold). As round 3 already shows excellent enrichment, it was decided to stop to prevent decrease in phage diversity.
| Phage Quantity(pfu)/well | Coating with antigen | Coating with antigen | Coating with antigen | Coating with buffer(negative control) | Coating with buffer(negative control) | Coating with buffer(negative control) |
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R3 | R2 | R3 | |
| 3.16*1011 | 2.85 | 2.35 | 2.91 | 0.04 | 0.03 | 0.03 |
| 1*1011 | 2.46 | 2.34 | 2.27 | 0.02 | 0.02 | 0.02 |
| 3.16*1010 | 1.84 | 2.75 | 2.29 | 0.02 | 0.02 | 0.02 |
| 1*1010 | 1.85 | 2.28 | 2.52 | 0.01 | 0.02 | 0.01 |
| 3.16*109 | 0.52 | 1.78 | 2.42 | 0.01 | 0.02 | 0.01 |
| 1*109 | 0.04 | 1.03 | 1.9 | 0.01 | 0.01 | 0.01 |
| 3.16*108 | 0.08 | 0.17 | 1.04 | 0.01 | 0.01 | 0.01 |
| 0 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
Data confirm significant enrichment as indicated by good specific binding + very low background (bold).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1.9 | 0.13 | 0.1 | 1.33 | 1.96 | 0 | 0.23 | 0.02 | 0.18 | 1.64 | 0 |
| B | 1.43 | 0 | 0 | 0 | 0 | -0.01 | 1.78 | 1.27 | 1.82 | 0.07 | 1.65 |
| C | 0 | 0 | 0 | 1.82 | 2.07 | -0.01 | 0.1 | 1.81 | 0.03 | 0 | 0 |
| D | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 | 0.01 | 2.19 | 0.03 | 0 |
| E | 0 | 0.02 | 0 | 0 | 1.74 | 0 | 0.05 | 0.04 | 0 | 1.29 | 0 |
| F | 1.5 | 0.96 | 1.03 | 0.44 | 0 | 2.27 | 0.17 | 1.46 | 0 | 0.01 | 0 |
| G | 1.74 | 0 | 1.15 | 0 | -0.01 | 0 | 1.78 | 0.02 | 0.01 | 1.27 | 0 |
| H | 0.01 | 0 | 0.01 | 0 | 1.11 | -0.01 | 0.21 | 1.48 | 1.29 | 1.04 | 0 |
30 clones are clearly positive (bold) and were sequenced, resulting in a total of 14 different unique sequences identified. All 14 sequences were delivered to customer. Our customer expressed the 14 antibodies as recombinant VHHs and tested them against cells overexpressing the protein of interest at their surface. Ten of them bound well to the cells with a clear shift,compared to control cell, of which 5 even had blocking activity.
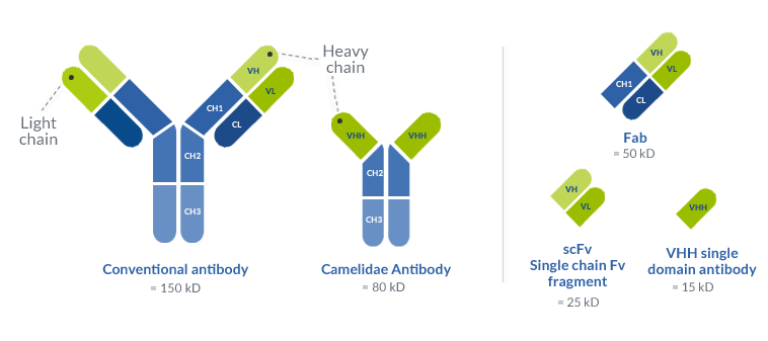
Conventionally we use IgG antibodies from mice, rat, rabbit etc. They have a size of 150kDa and are composed of 2 heavy and 2 light chains.
A group of researcher found that all members of the Camelidae family possess a special subclass IgG devoid of light chains; these antibodies consist of two heavy chains attached to variable domains (VHH).
Due to this, camelidae antibodies are much smaller, about 95kDa. The VHH alone, which is 15kDa, is also known as single domain antibody.
It is considered as the smallest naturally derived antigen-binding fragment, which is important for the development of several antibody drugs or diagnostic tools.
VHH antibodies present several characteristics and advantages making them a valuable tool that can be exploited in the research field
as well as in therapeutics and diagnostic applications. Some of these characteristics include:
VHH antibodies, also called “NANOBODY® proteins“, are single domain antibodies corresponding to the variable region of heavy chain of camelid anti-body. Thanks to their small size, 15 kDa, these molecules are featured by their ability to access to hidden epitopes and to easily associate with concave shaped proteins, like catalytic sites of enzymes, which are usually cryptic and not accessible to conventional antibodies.
Their small size also facilitates their penetration and clearance from tissues. VHH antibodies are also known as single domain structures which enables them to be expressed in cells without a need for supramolecular assembly and folding as it’s the case for immunoglobulins. Their small size, robustness and high stability make them effective tools for intracellular protein manipulation and analysis. In addition to their application in clinical and diagnosis fields, VHH have also paved the way for highly valuable applications in research and agriculture.
VHH-based tracing technology is highly suitable for tracking endogenous proteins of plants, allowing the visualization of endogenous cellular activities in plant cells. For instance, actin filaments can be visualized in tobacco cells thanks to actin chromobodies that label actin cytoskeleton.
Some knockdown strategies in plants are based on using VHH antibodies that can be targeted to a target protein to artificially alter its natural localization and thus, inhibit its function. VHH antibodies can also be used as intracellular “nanotraps”. For instance, GFP targeting VHH antibodies can be anchored at a distinct cellular compartment to trap GFP-tagged target protein.
VHH antibodies can be used for immunomodulation through ubiquitin-proteasome system (UPS), by fusing them to degrons, thus leading to targeting the protein of interest for ubiquitylation and proteasome-mediated degradation.
VHH antibodies are promising tools in plant resistance to invading and deadly pathogens. Ectopic expression of antigen specific VHH can establish resistance to some pathogens. The ability of VHH to bind some key adhesion proteins of pathogens prevent them from reproducing, moving and attaching to plants, and thus block their life cycle. VHH reduce also plant damage by neutralizing toxins secreted by some pathogens.
NANOBODY® compound is a registered trademark of Ablynx N.V.
