Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG4, kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Dostarlimab Biosimilar - Anti-PDCD1, PD1, CD279 mAb - Research Grade |
|---|---|
| Source | CAS 2022215-59-2 |
| Species | Humanized |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Dostarlimab,ANB-011,TSR-042,PDCD1, PD1, CD279,anti-PDCD1, PD1, CD279 |
| Reference | PX-TA1526 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG4, Kappa |
| Clonality | Monoclonal Antibody |
Dostarlimab is a humanized monoclonal antibody. It aims to block programmed death receptor -1 (PD1). Dostarlimab has been approved in 2021 in the treatment of endometrial cancer under the brand name Jemperli.
Programmed death receptor-1, is also called Programmed cell death protein (PDCD1). It is the cluster of differenciation 279 (CD279). This protein is an immune checkpoint, it has an inhibbitory activity on immune response. Indeed it can be found at the surface of immune T-cell. Its neutralization is therefore involved in many therapeutic research areas.
PD-1 has two ligands, PDL-1 and PDL-2. PDL1 are expressed at the surface of many cancer cells. Therefore they activate the negative response of PD1. The blockade of the interaction between PD1 and its ligand is thought to be a promissing treatment of cancers. In mouse models, PD-1 blockade, has been shown to improve CD8+ T cell function. Many monoclonal antibodies have been developped to target and neutralize the binding activity of PD1. In addition to Dostarlimab, Ipilimumab, Pembrolizumab and Nivolumab for example have shown good results.
In early June 2022, a phase 2 study reported good results in the application of Dostarlimab in rectal cancer treatment. Indeed a cohort of 12 patients has received the treatment for 6 months. At the end of the period, cancer cells were found in none of them. Besides, no side effect of grade 3 or higher were reported. This study raised a major interest in the scientific community.
Dostarlimab biosimilar is a humanized antibody produced in ProteoGenix proprietary mammalian cell line XtenCHOTM (Chinese Hamster Ovary -CHO). Proteogenix is using a recombinant DNA technology. Proteogenix is offering this product for research use only. Dostarlimab biosimilar is not suitable for clinical or therapeutic use.

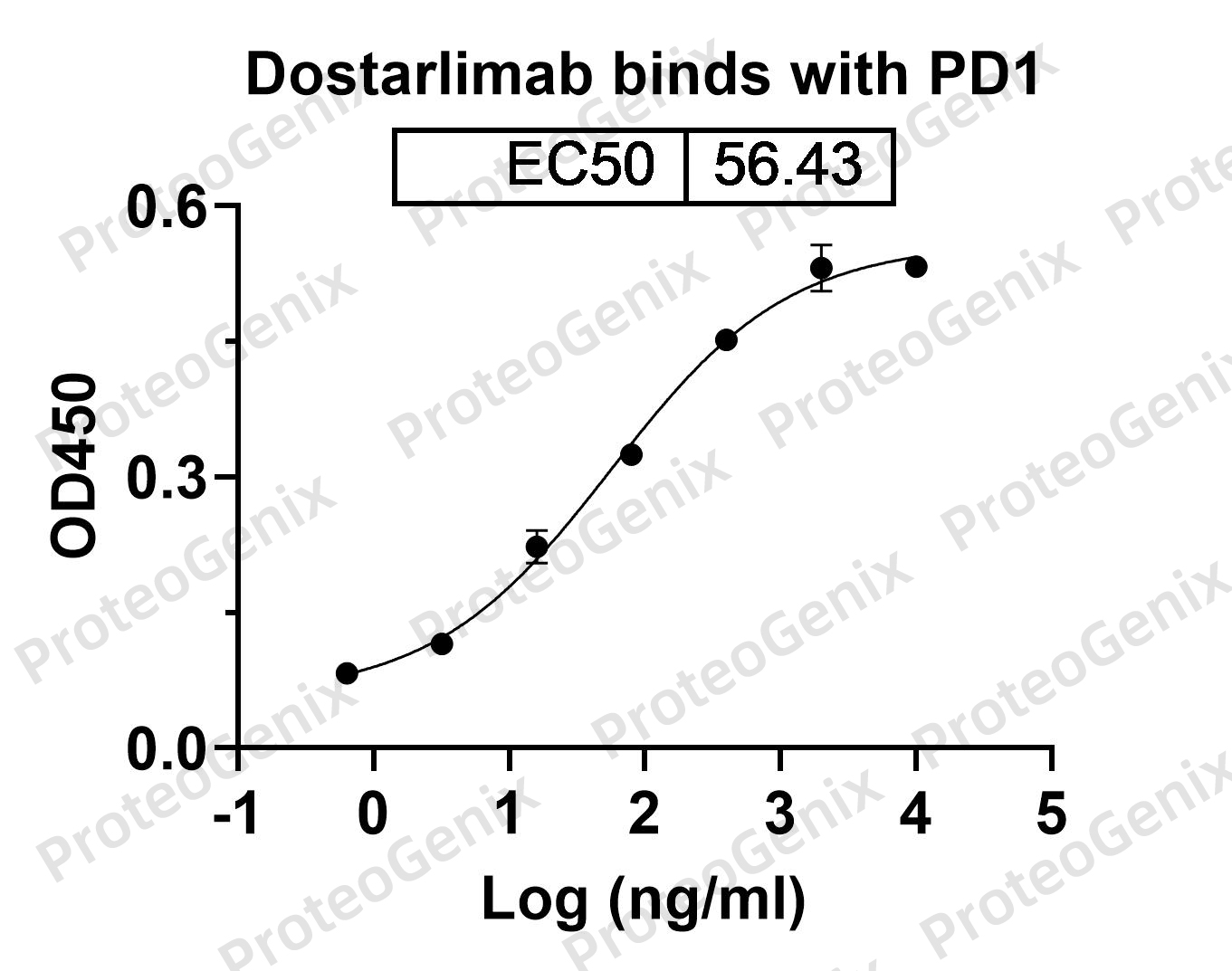
Immobilized CD279 Recombinant Protein (cat. No.PX-P4117) at 0.5µg/mL (100µL/well) can bind to Dostarlimab Biosimilar - Anti-PDCD1, PD1, CD279 mAb (cat. No.PX-TA1526) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Send us a message from the form below
Reviews
There are no reviews yet.