Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | (VH-CH2-CH3)2 |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Letolizumab Biosimilar - Anti-CD40LG, CD154 mAb - Research Grade |
|---|---|
| Source | CAS 1450981-87-9 |
| Species | Humanized |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Letolizumab,BMS-986004,CD40LG, CD154,anti-CD40LG, CD154 |
| Reference | PX-TA1459 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | (VH-CH2-CH3)2 |
| Clonality | Monoclonal Antibody |
Letolizumab Biosimilar, also known as Anti-CD40LG or CD154 monoclonal antibody, is a research grade therapeutic antibody that has shown promising results in preclinical studies for the treatment of various autoimmune and inflammatory diseases. In this article, we will discuss the structure, activity, and potential applications of Letolizumab Biosimilar.
Letolizumab Biosimilar is a monoclonal antibody that specifically targets CD40LG, also known as CD154. CD40LG is a cell surface protein that is primarily expressed on activated T cells and plays a crucial role in immune system regulation. Letolizumab Biosimilar is a fully humanized IgG1 antibody, meaning it is composed of human-derived amino acid sequences and has a molecular weight of approximately 150 kDa.
Letolizumab Biosimilar works by binding to CD40LG and blocking its interaction with its receptor, CD40, on immune cells. This prevents the activation of immune cells and the subsequent release of inflammatory cytokines, leading to a reduction in inflammation. Additionally, Letolizumab Biosimilar has been shown to induce regulatory T cells, which are important for maintaining immune tolerance and preventing autoimmune diseases.
Letolizumab Biosimilar has shown promising results in preclinical studies for the treatment of various autoimmune and inflammatory diseases. Some of the potential applications of Letolizumab Biosimilar include:
Rheumatoid Arthritis Rheumatoid arthritis (RA) is a chronic autoimmune disease that causes inflammation and damage to the joints. CD40LG has been implicated in the pathogenesis of RA, and Letolizumab Biosimilar has shown promising results in preclinical studies for the treatment of this condition. It has been shown to reduce inflammation and joint damage in animal models of RA.
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that can affect multiple organs and tissues. CD40LG has been found to be overexpressed in patients with SLE, and Letolizumab Biosimilar has shown potential in preclinical studies as a treatment for this condition. It has been shown to reduce inflammation and improve kidney function in animal models of SLE.
Inflammatory bowel disease (IBD), which includes conditions like Crohn’s disease and ulcerative colitis, is characterized by chronic inflammation of the gastrointestinal tract. CD40LG has been implicated in the pathogenesis of IBD, and Letolizumab Biosimilar has shown potential in preclinical studies for the treatment of this condition. It has been shown to reduce inflammation and improve gut barrier function in animal models of IBD.
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system. CD40LG has been found to play a role in the development and progression of MS, and Letolizumab Biosimilar has shown promise in preclinical studies as a potential treatment for this condition. It has been shown to reduce inflammation and improve neurological function in animal models of MS.
In conclusion, Letolizumab Biosimilar is a research grade therapeutic antibody that specifically targets CD40LG and has shown potential in preclinical studies for the treatment of various autoimmune and inflammatory diseases. Its mechanism of action involves blocking the interaction between CD40LG and its receptor, leading to a reduction in inflammation. Further clinical trials are needed to determine the safety and efficacy of Letolizumab Biosimilar in humans, but it holds promise as a potential treatment for a range of conditions.

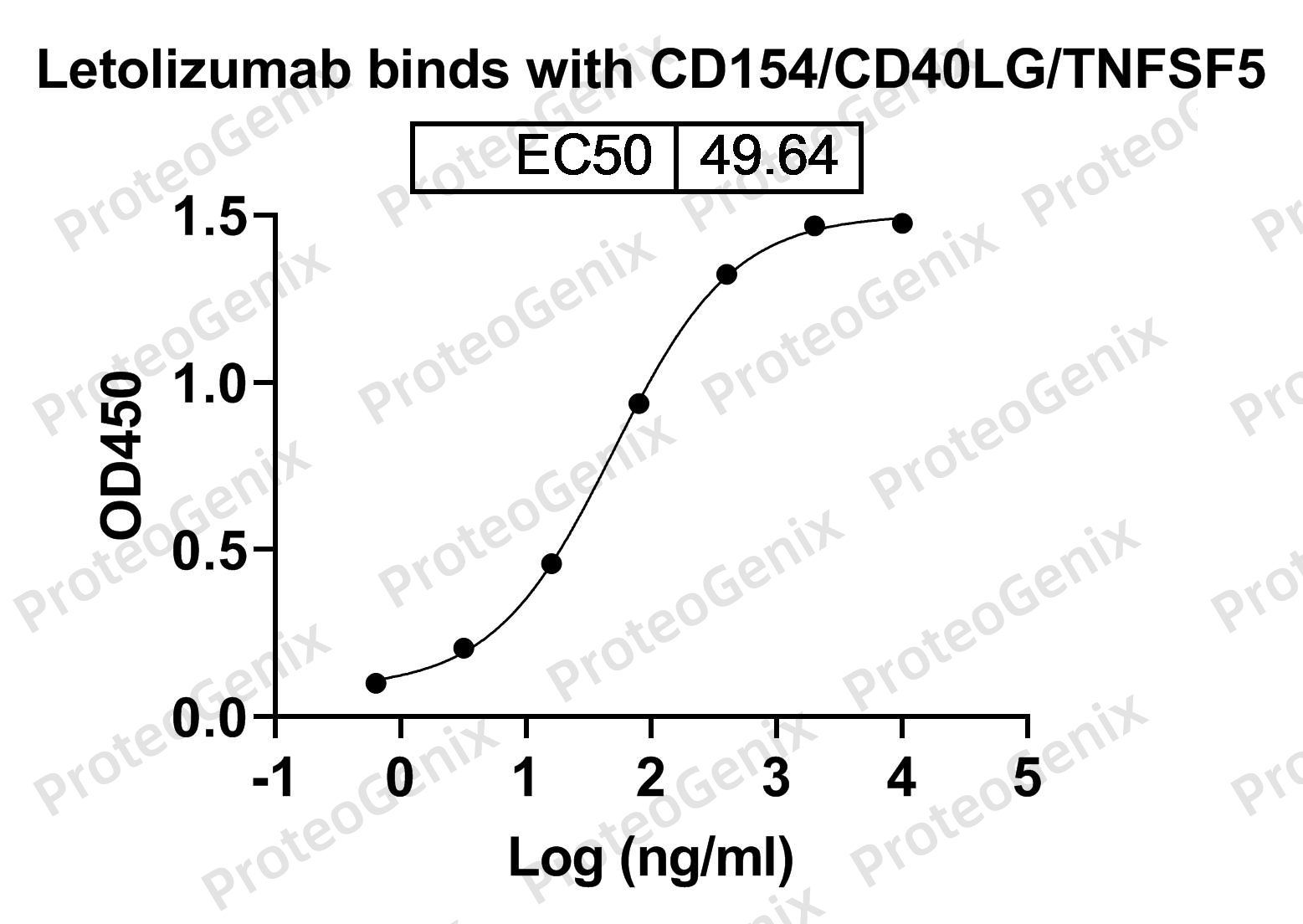
Immobilized CD40 ligand(CD40LG) (cat. No.PX-P4855) at 0.5µg/mL (100µL/well) can bind to Letolizumab Biosimilar - Anti-CD40LG, CD154 mAb (cat. No.PX-TA1459) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Send us a message from the form below
Reviews
There are no reviews yet.