Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG1, kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Sarilumab Biosimilar - Anti-IL-6R mAb - Research Grade |
|---|---|
| Source | DrugBank DB11767 |
| Species | Human |
| Expression system | Mammalian cells |
| Molecular weight | 150kDa |
| Purity | >85% |
| Buffer | PBS pH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | 4°C for short term; -20°C for long term |
| Brand | ProteoGenix |
| Aliases /Synonyms | Sarilumab,Sarilumab,IL-6R,anti-IL-6R |
| Reference | PX-TA1028 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG1, kappa |
| Clonality | Monoclonal Antibody |
The Sarilumab antibody is a fully human IgG1 kappa monoclonal protein targeting the human interleukin 6 receptor (IL-6R). It has been generated using the VelocImmune® platform by Regeneron Pharmaceuticals Inc. which consists of immunizing engineered mice modified to produce fully human antibodies instead of murine immunoglobulins. Sarilumab was granted approval by the Food and Drug Administration (FDA) in 2017 for the treatment of rheumatoid arthritis (RA), an autoimmune condition. RA is a long-term condition affecting hands, feet, and wrists often causing tiredness and weight loss.
Sarilumab and Tocilizumab (a humanized monoclonal antibody) share the same molecular target and mechanism of action. By competitively binding the IL-6R, both antibodies are known to disrupt the pro-inflammatory response caused by the interleukin 6 (IL-9) molecule, a small soluble glycoprotein known to be overexpressed in multiple inflammatory pathologies such as the cytokine release syndrome or “cytokine storm.” Both antibodies have proved their effectiveness as monoclonal antibody therapies in vitro and the clinic.
However, recent studies have shown that Sarilumab’s in vitro binding affinity is 15-22-fold higher than that of Tocilizumab. The improved affinity also translated into a better clinical efficiency suggesting that Sarilumab is capable of effectively blocking the IL-6R with a lower dose and dosage frequency than the ones recurrently necessary in Tocilizumab-based therapies. Consequently, Sarilumab may have an overall lower cost than its humanized counterpart for the treatment of multiple diseases.
The evaluation of Sarilumab as a COVID-19 treatment is currently under regulatory revision. Prior studies with this therapeutic antibody showed a marked improvement in patients in intensive care (over 89% of patients experienced a significant improvement after receiving the antibody intravenously). However, further studies are necessary to access the efficiency and safety of the fully human therapeutic antibody for blocking IL-6R in COVID-19 patients and arrest the onset of the acute respiratory distress syndrome (ARDS) shown to contribute to significant lung damage in the most severe cases of the disease. This product is for research use only.

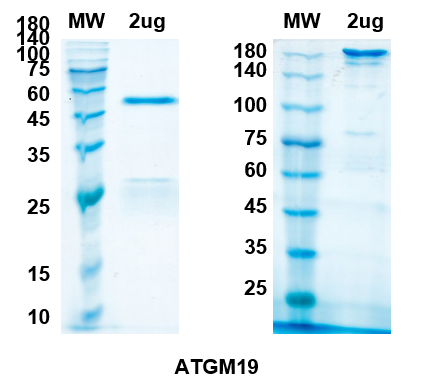
Sarilumab Biosimilar - Anti-IL-6R mAb, on SDS-PAGE under reducing and non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.

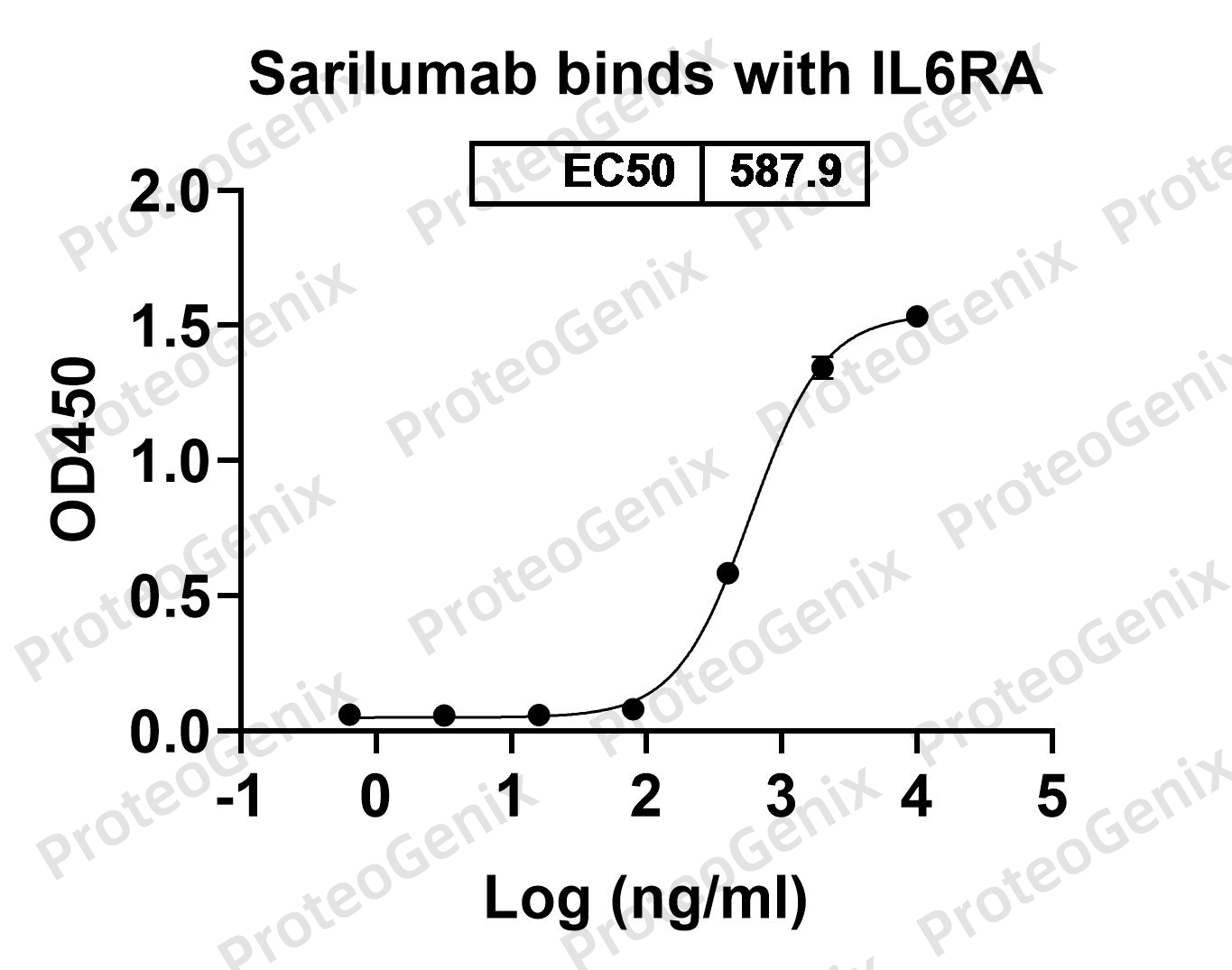
Immobilized CD126 Recombinant Protein (cat. No.PX-P4104) at 0.5µg/mL (100µL/well) can bind to Sarilumab Biosimilar - Anti-IL-6R mAb (cat. No.PX-TA1028) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450
Related products
Send us a message from the form below
Reviews
There are no reviews yet.