Cart (0 Items)
Your cart is currently empty.
View Products


Validated in other, ELISA
| Size | 100ug, 10mg, 1MG, 25mg |
|---|---|
| Isotype | IgG1, kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Nirsevimab Biosimilar - Anti-RSV mAb - Research Grade |
|---|---|
| Source | CAS 1989556-22-0 |
| Species | Humanized |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery lead time in business days | 3-5 days if in stock; 3-5 weeks if production needed |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Nirsevimab,MEDI-8897,RSV,anti-RSV |
| Reference | PX-TA1530 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG1, kappa |
Nirsevimab Biosimilar, also known as Anti-RSV mAb, is a novel monoclonal antibody (mAb) that has shown promising results in the treatment of respiratory syncytial virus (RSV) infections. This biosimilar is a research grade version of the original Nirsevimab, which is currently in clinical trials for the prevention of RSV infections in infants.
Nirsevimab Biosimilar is a recombinant humanized mAb that is produced in Chinese hamster ovary (CHO) cells. It is composed of two identical heavy chains and two identical light chains, each with a molecular weight of approximately 150 kDa. The heavy chains consist of four constant domains (CH1, CH2, CH3, and CH4) and one variable domain (VH), while the light chains have two constant domains (CL and CL2) and one variable domain (VL).
The variable domains of Nirsevimab Biosimilar are responsible for its specificity and binding to the RSV fusion (F) protein. This protein is essential for the virus to enter and infect host cells, making it a crucial therapeutic target for RSV infections.
Nirsevimab Biosimilar works by binding to the RSV F protein and preventing it from fusing with the host cell membrane. This inhibits the virus from entering the cell and replicating, ultimately stopping the infection from spreading. Additionally, the mAb also triggers an immune response that helps in clearing the virus from the body.
Nirsevimab Biosimilar has shown promising results in preclinical studies and is currently being evaluated in clinical trials for the prevention of RSV infections in infants. RSV is a common respiratory virus that can cause severe respiratory illness in young children, especially those with underlying health conditions. Currently, there is no approved vaccine or effective treatment for RSV, making Nirsevimab Biosimilar a potential game-changer in the field of respiratory infections.
Compared to other mAbs targeting RSV, Nirsevimab Biosimilar has several advantages. Firstly, it has a longer half-life, allowing for less frequent dosing. This is particularly beneficial for infants who are at high risk of RSV infections and may require multiple doses during the RSV season. Secondly, it has a higher binding affinity to the RSV F protein, making it more effective in preventing viral entry into host cells. Lastly, Nirsevimab Biosimilar has a favorable safety profile, with no serious adverse events reported in clinical trials so far.
In conclusion, Nirsevimab Biosimilar is a promising therapeutic option for the prevention of RSV infections in infants. Its unique structure, mechanism of action, and potential advantages make it a highly sought-after treatment in the field of respiratory infections. With ongoing clinical trials, we can hope to see this biosimilar being approved for use in the near future, providing a much-needed solution for the prevention of RSV infections.

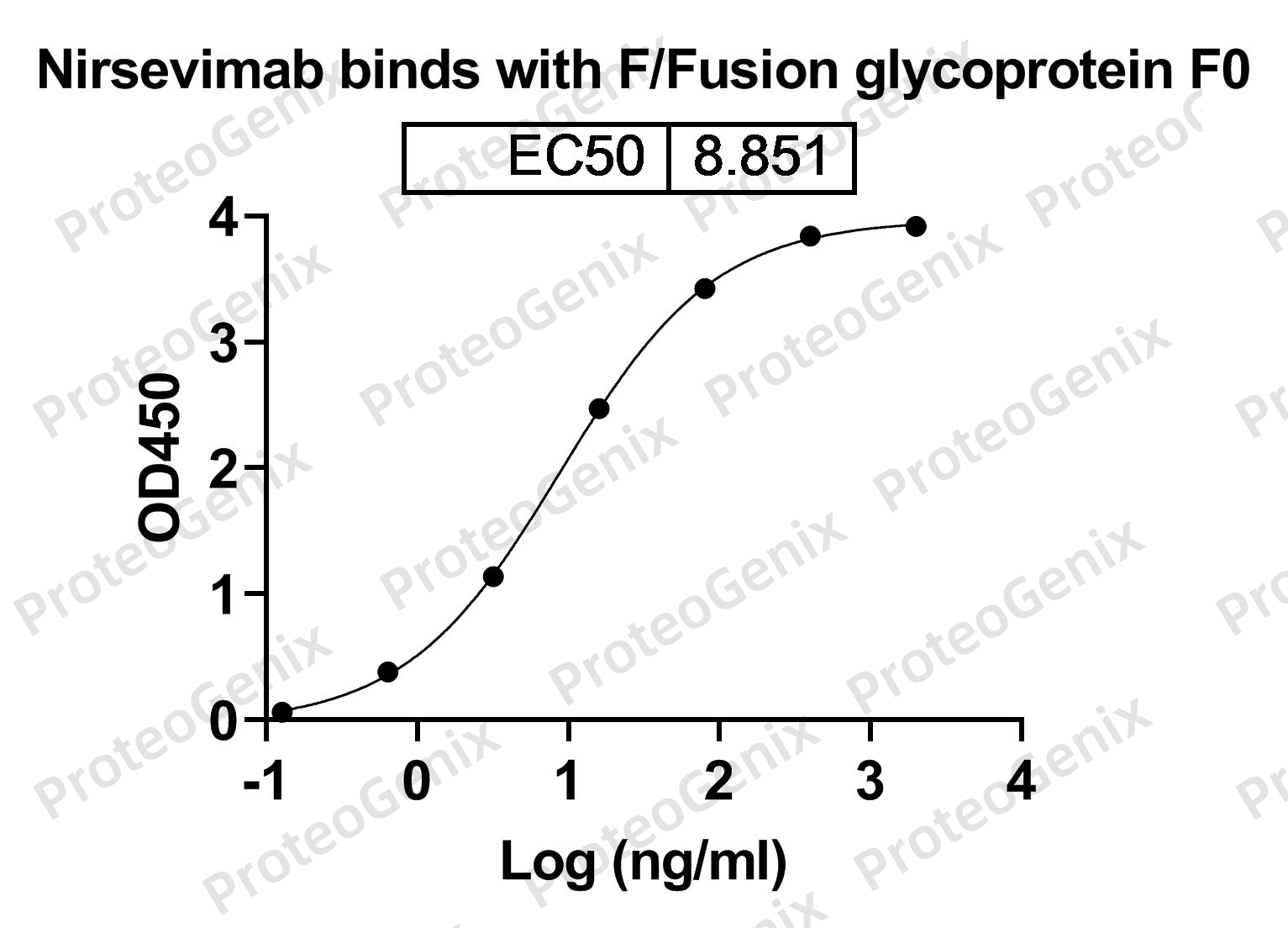
Immobilized HRSV-A2 Pre-F-Fusion glycoprotein F0 recombinant protein (cat. No.PX-P6126) at 0.5µg/mL (100µL/well) can bind to Nirsevimab Biosimilar - Anti-Fusion glycoprotein F0 mAb (cat. No.PX-TA1530) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450

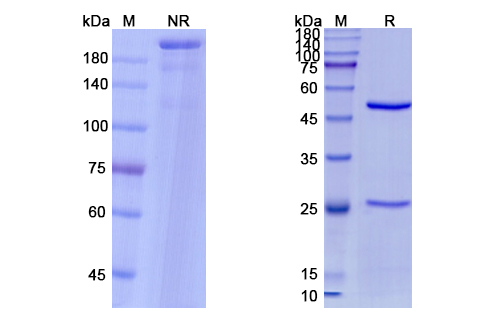
Nirsevimab Biosimilar - Anti-Fusion glycoprotein F0 mAb, on SDS-PAGE under reducing and non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.
Related products
Send us a message from the form below
Taylor Slade-Adjei –
In which application did you use the antibody?: ELISA
Did it work in your application?: Yes
The shipping and customer service is always exceptional!
jurata-orders –
In which application did you use the antibody?: other
Did it work in your application?: Yes
Solid mAb choice for assay development.
Denise Reaves –
In which application did you use the antibody?: other
Did it work in your application?: No
It’s pretty weak, recommend high concentrations when using in neutralization assay