Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | Bispecific Single Domains (VH-VH'-CH), IgG1na;na |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | XtenCHO |
| Applications | Elisa, WB |
| Product name | Erfonrilimab Biosimilar - Anti-CD274;CTLA4 mAb - Research Grade |
|---|---|
| Source | CAS 2367013-69-0 |
| Species | Humanized |
| Expression system | XtenCHO |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3 week if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Erfonrilimab,ERFONRILIMAB, ERFONRILIMAB, KN046,CD274;CTLA4,anti-CD274;CTLA5 |
| Reference | PX-TA1665 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | Bispecific Single Domains (VH-VH'-CH),IgG1na;na |
| Clonality | Monoclonal Antibody |
Erfonrilimab Biosimilar – Anti-CD274,CTLA4 mAb – Research Grade: A Potent Antibody Targeting Immune Checkpoint Proteins Erfonrilimab Biosimilar, also known as Anti-CD274,CTLA4 mAb, is a research grade monoclonal antibody that has gained significant attention in the field of immunotherapy. It is a biosimilar version of the FDA-approved drug, Nivolumab, and is designed to target two important immune checkpoint proteins, CD274 (also known as PD-L1) and CTLA4. This antibody has shown promising results in pre-clinical studies and is currently being evaluated in clinical trials for the treatment of various types of cancer.
Erfonrilimab Biosimilar is a fully human IgG4 monoclonal antibody, meaning it is derived from human cells and has four subclasses of immunoglobulin G. It is composed of two heavy chains and two light chains, connected by disulfide bonds. The antibody has a molecular weight of approximately 150 kDa and a half-life of 12-14 days in the human body.
The antibody has a specific binding site for CD274 and CTLA4, which are both immune checkpoint proteins expressed on the surface of immune cells. The binding of Erfonrilimab Biosimilar to these proteins inhibits their interaction with their respective receptors, PD-1 and CD80/86, leading to the activation of T cells and enhanced anti-tumor immune response.
Erfonrilimab Biosimilar has been shown to have potent anti-tumor activity in pre-clinical studies. By targeting CD274 and CTLA4, it blocks the inhibitory signals that cancer cells use to evade the immune system. This allows the immune system to recognize and attack cancer cells, leading to tumor regression.
Additionally, Erfonrilimab Biosimilar has also been shown to enhance the activity of other immune cells, such as natural killer cells and macrophages, further contributing to its anti-tumor effects. It has also been found to have a synergistic effect when combined with other immunotherapies, such as anti-PD-1 or anti-CTLA4 antibodies.
Erfonrilimab Biosimilar is currently being evaluated in clinical trials for the treatment of various types of cancer, including lung cancer, melanoma, and renal cell carcinoma. It has also shown potential in the treatment of other types of cancer, such as head and neck cancer, bladder cancer, and lymphoma.
The antibody is administered intravenously and is typically given every 2-3 weeks. It has shown promising results in both monotherapy and combination therapy settings, with manageable side effects.
Erfonrilimab Biosimilar, also known as Anti-CD274,CTLA4 mAb, is a research grade monoclonal antibody that has shown promising results in pre-clinical studies and is currently being evaluated in clinical trials for the treatment of various types of cancer. Its unique mechanism of action, targeting two important immune checkpoint proteins, CD274 and CTLA4, makes it a promising candidate for cancer immunotherapy. With further research and development, this antibody has the potential to improve outcomes for cancer patients and revolutionize the field of immunotherapy.

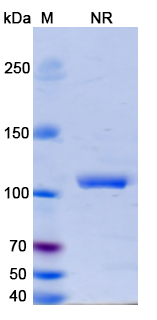
Erfonrilimab Biosimilar - Anti-CD274;CTLA4 mAb, on SDS-PAGE under non-reducing condition. The gel was stained overnight with Coomassie Blue. The purity of the antibody is greater than 95%.
Related products
Send us a message from the form below
Reviews
There are no reviews yet.