Cart (0 Items)
Your cart is currently empty.
View Products


| Size | 100ug, 1MG |
|---|---|
| Isotype | IgG2-G4-kappa |
| Brand | ProteoGenix |
| Product type | Primary Antibodies |
| Clonality | Monoclonal Antibody |
| Expression system | Mammalian cells |
| Applications | Elisa, WB |
| Product name | Ravulizumab Biosimilar - Anti-C5 mAb - Research Grade |
|---|---|
| Source | CAS 1803171-55-2 |
| Species | Humanized |
| Purity | >85% |
| Buffer | PBS buffer PH7.5 |
| Delivery condition | Blue ice (+4°C) |
| Delivery Time | 3-5 days if in stock; 3-5 weeks if production needed |
| Storage condition | store at -80°C |
| Brand | ProteoGenix |
| Aliases /Synonyms | Ravulizumab,ALXN-1210,ravulizumab-cwvz,C5,anti-C5 |
| Reference | PX-TA1452 |
| Note | For research use only. Not suitable for clinical or therapeutic use. |
| Isotype | IgG2-G4-kappa |
| Clonality | Monoclonal Antibody |
Ravulizumab Biosimilar, also known as Anti-C5 mAb, is a monoclonal antibody that targets the complement protein C5. It is a research-grade version of the FDA-approved drug, Ravulizumab, which is used for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). In this article, we will discuss the structure, activity, and potential applications of Ravulizumab Biosimilar as a therapeutic antibody.
Ravulizumab Biosimilar is a fully humanized IgG2/4 monoclonal antibody with a molecular weight of approximately 148 kDa. It is composed of two heavy chains and two light chains, linked together by disulfide bonds. The antibody has a Y-shaped structure, with two antigen-binding Fab regions at the end of each arm and a constant Fc region at the base. The Fab regions are responsible for binding to the complement protein C5, while the Fc region mediates effector functions such as complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.
Ravulizumab Biosimilar works by specifically binding to the complement protein C5 and inhibiting its activity. C5 is a crucial component of the complement system, which is a part of the immune system responsible for defending the body against pathogens. When activated, C5 triggers a cascade of reactions that leads to the formation of the membrane attack complex (MAC), a pore-forming complex that can damage cells and cause inflammation. By blocking the activity of C5, Ravulizumab Biosimilar prevents the formation of MAC and reduces the inflammatory response.
Ravulizumab Biosimilar has potential applications in the treatment of various complement-mediated diseases. As mentioned earlier, the FDA-approved version of this antibody is used for the treatment of PNH and aHUS. PNH is a rare blood disorder characterized by the destruction of red blood cells, while aHUS is a rare kidney disease caused by abnormal activation of the complement system. By inhibiting the activity of C5, Ravulizumab Biosimilar can improve the symptoms and outcomes of these diseases.
In addition to PNH and aHUS, Ravulizumab Biosimilar is being investigated for its potential in other complement-mediated diseases such as paroxysmal cold hemoglobinuria, myasthenia gravis, and neuromyelitis optica spectrum disorder. These diseases are also characterized by abnormal activation of the complement system and can benefit from the inhibitory effects of Ravulizumab Biosimilar.
In conclusion, Ravulizumab Biosimilar is a research-grade version of the FDA-approved drug, Ravulizumab, which targets the complement protein C5. It has a Y-shaped structure and works by inhibiting the activity of C5, thus preventing the formation of the membrane attack complex and reducing inflammation. This antibody has potential applications in the treatment of various complement-mediated diseases, including PNH, aHUS, paroxysmal cold hemoglobinuria, myasthenia gravis, and neuromyelitis optica spectrum disorder. Further research and clinical trials are needed to fully understand the potential of Ravulizumab Biosimilar as a therapeutic antibody.

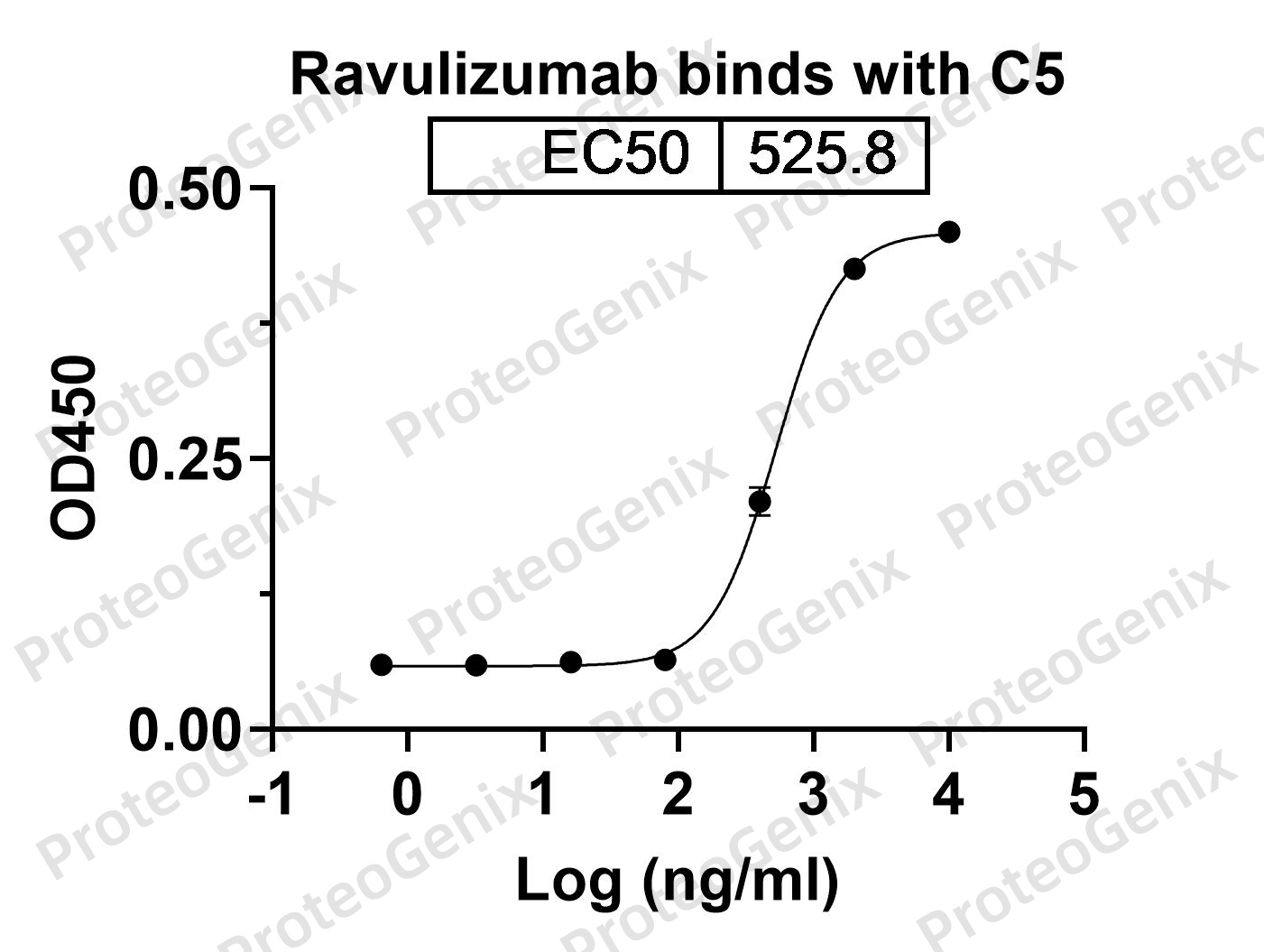
Immobilized Human C5 recombinant protein (cat. No. PX-P5117) at 0.5µg/mL (100µL/well) can bind Ravulizumab Biosimilar - Anti-C5 mAb (cat. No. PX-TA1452) in indirect ELISA with Goat Anti-Human IgG secondary antibody coupled with HRP measured by OD450 giving an EC50 at 525.8M.
Related products
Send us a message from the form below
Reviews
There are no reviews yet.