 Antibody production
Antibody production
Everything you need to know about the structure and types of antibodies in the forefront of the immune response
Antibodies are one of the most efficient biotherapies used for the treatment and prevention of diseases. But what do we know about these molecules and their role during the immune response? In this article, we explore the different types of antibodies produced by our organism in response to foreign molecules and the importance of understanding their structure to design better immunotherapies. Check out other frequently asked questions (FAQs) page about monoclonal antibodies on our dedicated page.
The underlying primary structure for all types of antibodies
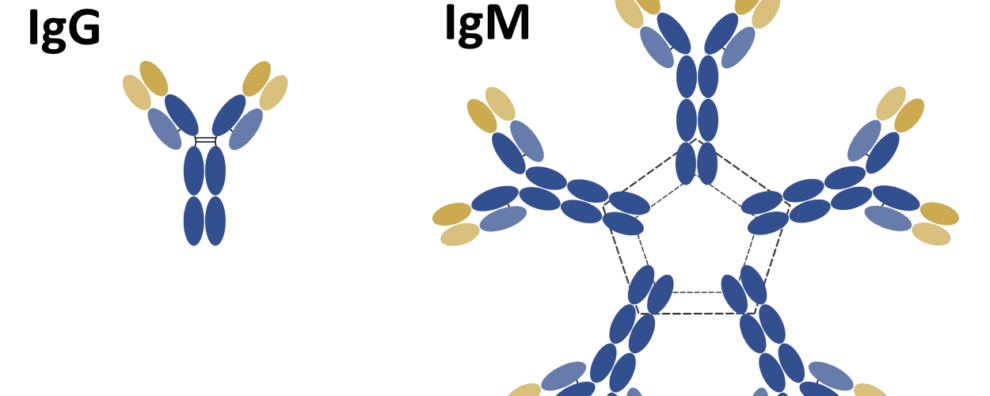
When we think of monoclonal antibodies, it’s the IgG isotype that comes to mind. This isotype was the first to be fully elucidated, due to its high levels of abundance and ease of isolation from serum.
The IgG isoform is a monomeric immunoglobulin consisting of two heavy (H) chains and two light (L) chains. The interaction between these chains confers these molecules the canonical “Y” shaped structure that makes a bridge between foreign antigens and our immune system.
The primary structure of V and H chains consist of an NH2-terminal variable domains (V) and one or more COOH-terminal constant domains (C). L chains contain a single V domain (VL) linked to a single C domain (CL) by disulfide bonds. While the H chain contains a VH and a CH1 regions linked by a flexible hinge sequence to two additional C domains (CH2 and CH3).
Early studies showed that the hinge region could be cleaved by papain, a protease that is still in use to produce antibody fragments for countless applications.
Papain can cleave immunoglobulins into:
- Two identical fragments containing the V domains of both the L and H chain, VL and VH respectively, and the first C domains of these two chains, CL and CH1 respectively
- One fragment containing the remaining C domains, CH2 and CH3
Interestingly, these fragments retained biological activity.
The two identical fragments were found to be responsible for antigen binding, thus they were termed Fab fragments. On the contrary, the third fragment could not bind to antigens, but it was readily crystallizable, earning the term of Fc fragment.
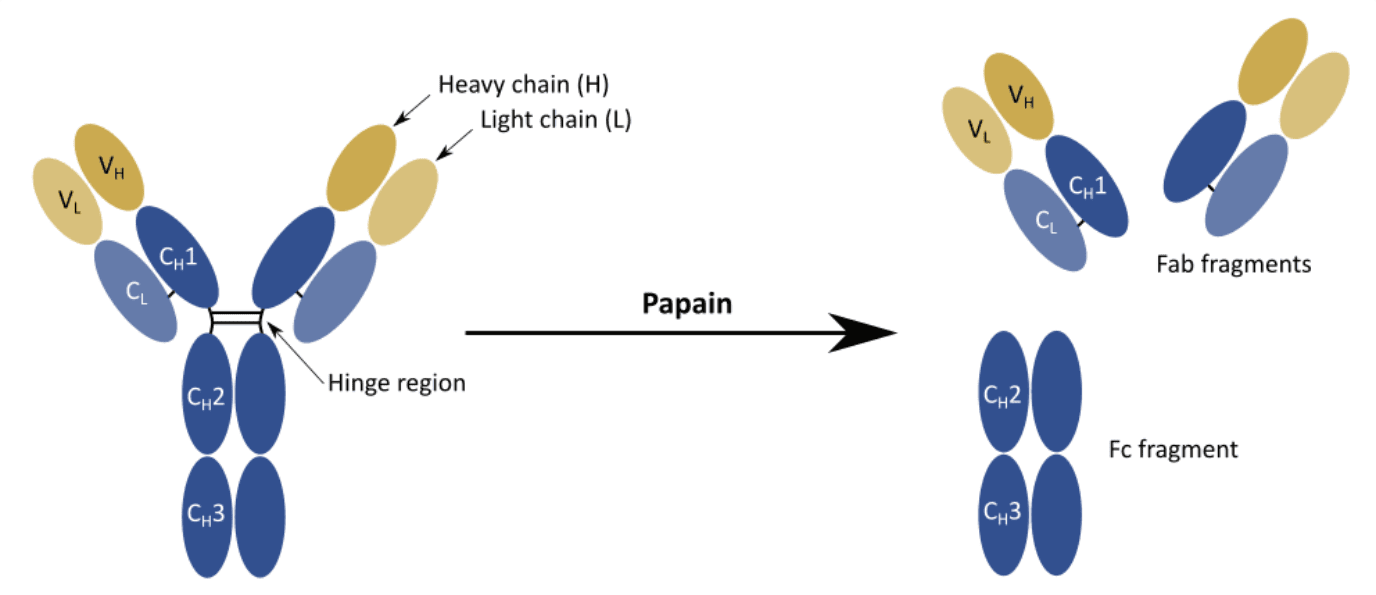
Despite not being fundamental for the interaction with the antigen, the Fc region was soon found to be responsible for effector functions such as interaction with the receptors located on the surface of immune cells (i.e. phagocytes) or activation of the complement cascade.
Within the V domain, each chain contains three hypervariable loops with surface complementarity to a specific antigen. Thus, they were named complementarity determining regions (CDRs). The four additional regions between the three CDRs loops were found to be more conserved and thus were termed framework regions (FRs).
The identification of these precise regions propelled the development of many early antibody engineering techniques.
Isotypes and subtypes of antibodies
IgG structure is the most well-known type of immunoglobulin because it is the most abundantly expressed and secreted by our organism. For this reason, molecules from the IgG isotype also became the preferred format used for the development of therapeutic antibodies.
Nevertheless, IgG is only one of five isotypes playing an important role in our immune system. The other four isotypes differ from IgG in terms of structure, sequence, number of constant domains, form, and role.
Main isotypes and subtypes of antibodies found in humans. MW – molecular weight.
| Isotype | IgM | IgD | IgG | IgA | IgE |
|---|---|---|---|---|---|
| Common structure | Pentamer | Monomer | Monomer | Monomer
Dimer |
Monomer |
| Subtypes | None | None | 4 (IgG1-4) | 2 (IgA1-2) | None |
| H chain type | µ | δ | γ | α | ε |
| H chain MW (kDa) | 70 | 60 | 50 (IgG1, 2, 4)
60 (IgG3) |
55 | 70 |
| L chain MW (kDa) | 23 | 23 | 23 | 23 | 23 |
| Total MW (kDa) | 970 | 180 | 150-170 | 165 (monomer)
350 (dimer) |
190 |
| Relative abundance | 5-10% | <1% | 70-85% | 5-15% | 0.002% |
| Serum half-life | 5 days | 3 days | 23 days | 6 days | 2.5 days |
| Complement activation | Yes | No | Yes (except IgG4) | No | No |
| Cross placenta | No | No | Yes | No | No |
| Function | Primary response | Homeostasis | Secondary response
Neutralization of toxins and virus |
Primary response | Homeostasis |
Structurally, significant differences can be found among the different isotypes. For instance, the H chain of IgG, IgD, and IgA consists of 3 C domains (CH1-3) and a hinge region between the CH1 and CH2 domains.
Interestingly, antibodies from the IgM and IgE isotypes lack a hinge region, but each H chain contains an additional C domain (CH1-4).
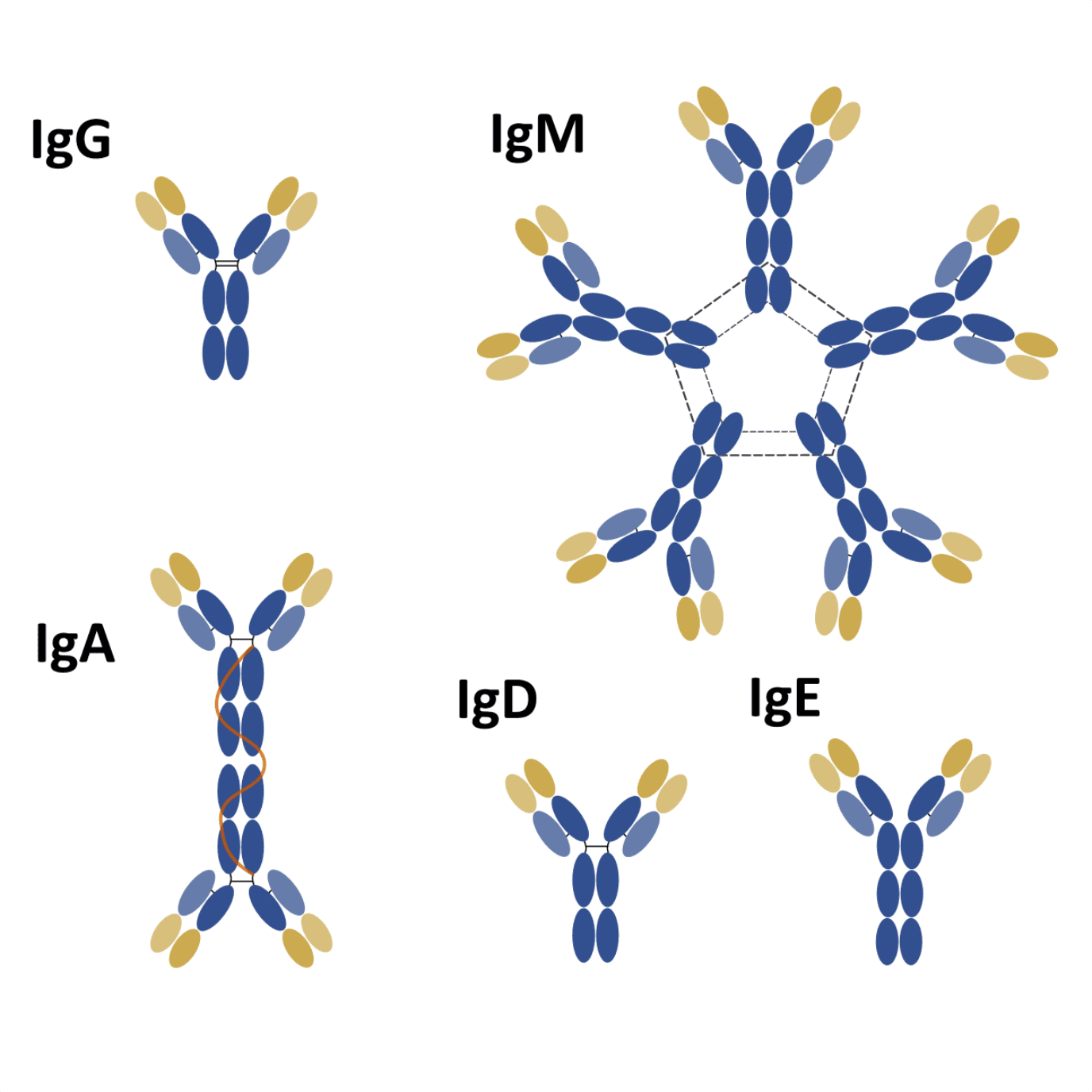
Types of antibodies and their role in the immune response
IgM is the first isotype to be expressed during B cell development. In the early stages of development, only antibodies from the IgM isotype coat the surface of a B cell. But as soon as it leaves the bone marrow and migrates to the peripheral lymphoid organs (i.e. spleen, lymph nodes or intestinal mucosa tissue), the B cell starts expressing a second isotype on its surface – IgD.
The co-expression of these two isotypes during the early immune response continues to be enigmatic. However, most studies seem to point out that IgD is a regulatory molecule implicated in B cell development and homeostasis.
When a B cell is activated by an antigen, it proliferates, and during this process, the C domains of the H chains of their antibodies, undergo extensive genetic recombination. This process is called class switching (or isotype switching), and it allows the cell to preserve antigen-specificity and, at the same time, change the effector function of the immunoglobulins.
After activation and switching, B cell ceases to express IgM and IgD and starts expressing IgG, IgE or IgA. Each isotype is produced as a response to different conditions. IgG is the main key player of the secondary immune response, and it is secreted to the bloodstream and extracellular fluids becoming the most abundant isotype in the human serum.
Interestingly, each subtype of the IgG class can be associated with a different type of response. For instance, IgG1 and IgG3 are usually produced as a response to protein antigens, while IgG2 and IgG4 are produced as a response to polysaccharide antigens.
On the contrary, IgA is the main isotype found in secretions like saliva, tears, and milk, and in the mucous epithelium of the intestinal and respiratory tracts. While IgG is extremely efficient at opsonizing pathogens for digestion by phagocytes and activation of the complement system, IgA is a less efficient opsonization agent and weaker activator of the complement cascade.
However, these differences are far from being surprising. IgG operates in the tissues and blood, where accessory cells and molecules are available, while IgA operates in the epithelium and secretions where complement and phagocytes are scarce. Thus, while IgG works mainly as a mediator between the antigen and the immune system, IgA molecules work directly as neutralizing agents in the organism’s first line of defense.
IgE antibodies can be found at very low levels and exclusively beneath the skin, mucosa and in the connective tissues of blood vessels. Despite its low abundance, molecules from the IgE isotype are able to trigger the most intense inflammatory responses.
They do so due to their ability to sensitize mast cells, basophils and activated eosinophils in an antigen-dependent way. This process causes the production and release of histamine and other powerful inflammatory mediators of the allergenic response.
Types of antibodies and challenges in passive immunization
IgG is considered the major player of the secondary immune response.
It is the isotype with the longest half-life and lowest molecular weight among all other immunoglobulins. Thus, making it the optimal candidate for the design of successful passive immunization strategies.
However, IgG is mainly produced in the bloodstream and tissues. This means that in order for passive immunization to work it is essential to optimize the antibody delivery strategy.
In fact, antibodies undergo irreversible denaturation in the acidic environment of the digestive system and they are only weakly absorbed by the epithelium. For these reasons, most antibody therapies are delivered through the intravenous administration route (IV).
This type of injection allows the rapid delivery of antibodies to the systemic circulation, which subsequently leads to a higher bioavailability of antibodies in the patient’s serum.
But the efficiency of IV delivery comes with a cost. IV injections require hospitalization and, for this reason, they quickly escalate the costs associated with antibody therapy.
Some antibodies can be administrated by extravascular injections, and still achieve therapeutic concentrations in the organism. However, many researchers agree that the simplest and most optimal solution would be developing antibody delivery strategies that allow oral administration.
It was Francis Brambell who first allows us a glimpse into our organism’s solution to this obstacle. He found it after investigating how mothers were able to transfer antibodies to their infants.
His research led to the identification of the neonatal Fc receptor (FcRn), a protein able to bind and protect the IgG molecules in a pH-dependent manner.
Encoded by the FCGRT gene in humans, this factor associates with the IgG Fc region in a non-covalent way. At lower pH (pH 6.0), this factor binds tightly to the Fc region, but the interaction ceases once the antibody reaches an environment with physiological pH (pH 7.4).
Interestingly, FcRn receptors are expressed in both neonates and adults, albeit at different levels. Nevertheless, the presence of these receptors in the epithelium of lungs and kidneys in adults has long made FcRn-IgG complexes be regarded as a potential solution for oral delivery of therapeutic antibodies.
Unfortunately, promising results obtained using in vitro cultured cells, have yet to translate to animal models. And due to the disappointing initial results, research using this approach has become scarce. Nevertheless, FcRn-IgG complexes were still found to be useful for the treatment of intestinal diseases.
Concluding remarks
Antibodies are complex molecules and key players in our organism’s immune response. Although IgG is the most widely used and discussed isotype of the immunoglobulin superfamily, it is not the only isotype playing an important role in our organism.
In fact, the immune response starts with the expression of IgM and IgD isotypes on the surface of a B cell. As soon as this cell is challenged by an antigen, the IgM and IgD undergo class switching and differentiate into one of the three isotypes known to play a role in the secondary immune response: IgA, IgG and IgE.
All of these antibodies share a similar core structure and, for this reason, face similar challenges in terms of drug delivery. Antibodies are prone to irreversible denaturation in the digestive system’s acidic environment, for this reason, most antibodies need to be delivered directly into the patient’s systemic circulation. This increases treatment costs significantly.
Many scientists attempted to solve this limitation by conjugating antibodies with a well-characterized protector molecule – FcRn. However, early promising results were still not replicated in animal models. Hence, most scientists still consider vital to find new cost-effective solutions for antibody delivery.
- Brambell, F. W. R. The passive immunity of the young mammal. Biol Rev. 1958; 33:488–531. doi: 10.1111/j.1469-185X.1958.tb01412.x
- Geisberger, R. et al. The riddle of the dual expression of IgM and IgD. Immunology. 2006; 118(4): 429–437. doi: 10.1111/j.1365-2567.2006.02386.x
- Janeway, C.A. et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10757/
- Lobo, E. D. et al. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004; 93(11):2645-2668. doi: 10.1002/jps.20178
- Muzammil, S. et al. FcRn binding is not sufficient for achieving systemic therapeutic levels of immunoglobulin G after oral delivery of enteric‐coated capsules in cynomolgus macaques. Pharmacol Res Perspect. 2016; 4(3): e00218. doi: 10.1002/prp2.218
- Pyzik, M. et al. The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol. 2019; 10:1540. doi: 10.3389/fimmu.2019.01540
- Schroeder, H. W. Jr, and Cavacini, L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010; 125(202): S41–S52. doi: 10.1016/j.jaci.2009.09.046
