 Antibody production
Antibody production
Antibody production: rabbit versus mouse, which host best fits your project?
In recent years, rabbit monoclonal antibody production using phage display has been sought after by researchers and industry leaders around the world as a suitable alternative to rodent-derived antibodies. What has motivated this shift towards a different species? What are the advantages of using rabbit versus mouse-derived antibodies? And which techniques are currently employed to generate a continuous supply of these bioreagents?
Why antibody production in rabbits became so popular?
Mice have been the dominant host for antibody production since the discovery of the hybridoma technology in the 1970s. But, as researchers expanded our knowledge on the immune system of different species, other hosts have attracted attention due to their unique characteristics.
Rabbits were the first of these hosts to captivate scientist’s attention. Unlike rodent’s immune systems, rabbits seemed to be able to recognize a much broader diversity of antigens. Interestingly, antibodies produced by rabbits also tend to exhibit a significantly higher affinity, especially against epitopes of human origin, or epitopes known to be non-immunogenic in mice.
These characteristics, allied to the larger size of rabbits in comparison to mice, made these hosts desirable for the production of antibodies for research, analytics, and diagnostics.
The immune response and antibody production in rabbits
Like the human immune response, antibody production in rabbits is mediated by the complex interaction between antigen-presenting cells (APC), T cells, and B cells which, in turn, differentiate into antibody-secreting plasma cells.
The primary immune response leads to the production of the IgM isotype, and later shifts to the secretion of IgG (up to 20 mg/ml) or IgA (up to 4 mg/ml). Up to now, no homolog has been found for the IgD isotype in rabbits. Moreover, in contrast with other species, rabbit IgG isotype does not differentiate into different subclasses.
Although the canonical structure of rabbit antibodies resembles that of mouse and human antibodies, there are some important differences in terms of composition. For instance, rabbit IgG generally has fewer amino acid residues at the N terminus and in the D-E loop. Moreover, these biomolecules also possess an unusual interdomain disulfide bond. This bond, scientists believe, may play a crucial role in increasing the stability and extending the shelf-life of these molecules.
Moreover, the CDR3-loop (third complementarity-determining region) of the light chain was found to be significantly longer in rabbits in comparison to its human and mouse counterparts. This characteristic may also contribute to the increased binding strength of these bioreagents.
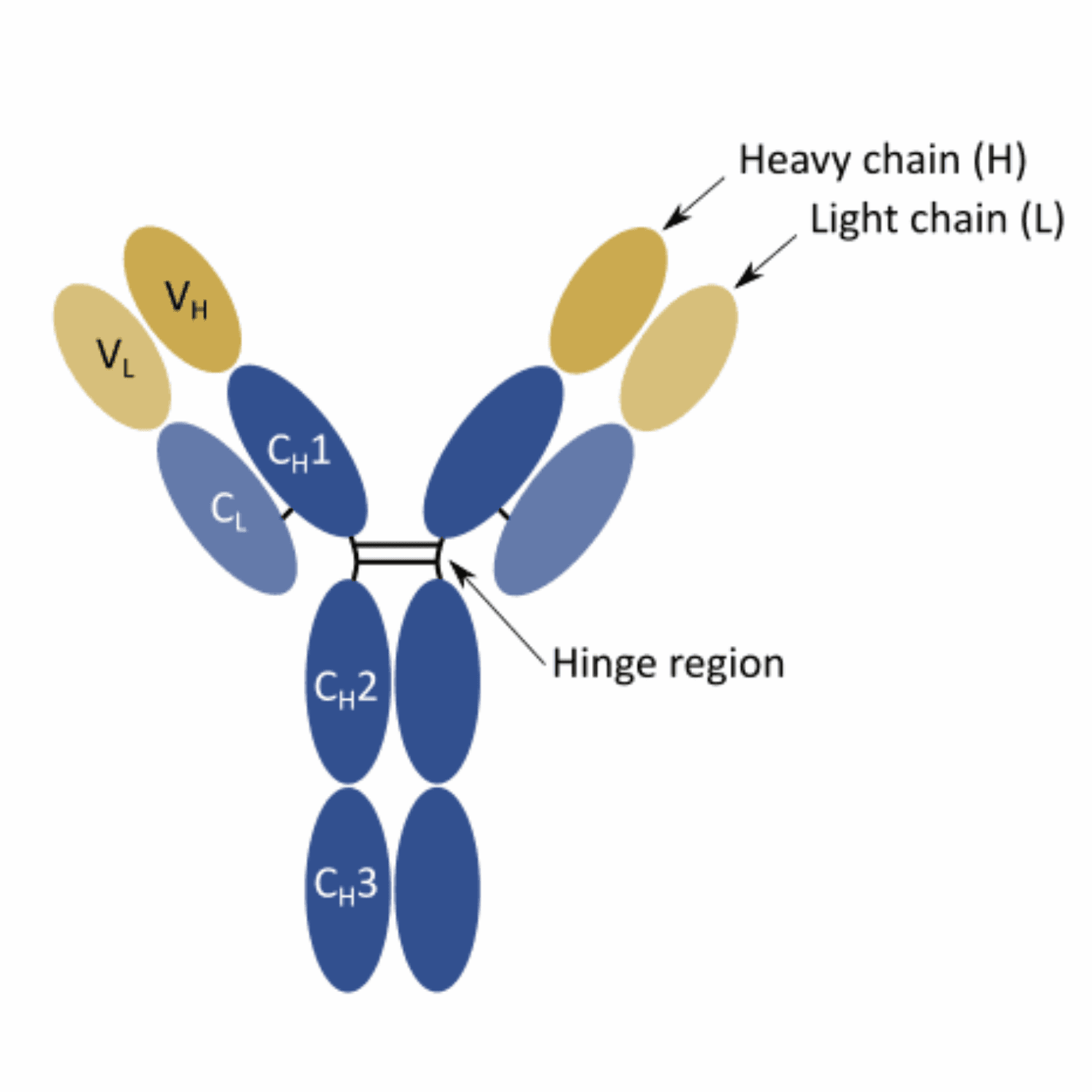
Beyond the structural differences, rabbits generate their antibody repertoires using significantly different mechanisms than the ones described for humans and rodents. Moreover, the diversification of the rabbit’s naïve repertoire is significantly affected by age and the growth conditions. For instance, new-born rabbits are relatively immuno-incompetent. Plus, rabbits raised in a germfree environment are also found to have relatively limited primary antibody diversification.
Unlike humans and rodents, the VH repertoire of rabbits is more limited, and the VL repertoire is significantly more diverse. However, the limited VH repertoire is often compensated by significantly higher rates of gene conversion and somatic hypermutation in these hosts.
Polyclonal antibody production in rabbit versus mouse
For analytical essays and diagnostics, rabbit polyclonal antibodies are usually preferred over rodent polyclonal antibodies. This preference stems from three main reasons, the first relates to the physiological size of rabbits. Since these hosts are considerably larger than typical rodents used in antibody generation, the quantity of antisera they can produce is also significantly higher.
Moreover, rabbits can generate antibodies against antigens that often fail to elicit an immune response in rodents. And finally, rabbits have a proven track record of generating antisera containing a high titer of antibodies with increased sensitivity and affinity towards a defined target.
Monoclonal antibody production in rabbit
Hybridoma technology
Polyclonal rabbit antibodies are still invaluable reagents. However, like conventional rodent-derived polyclonal antibodies, these reagents also suffer from high batch-to-batch variability. Hence, for applications requiring high consistency and reproducibility, monoclonal antibodies should be used instead.
The generation of rabbit monoclonal antibodies is significantly more challenging than the generation of their murine counterparts. Early attempts to obtain these antibodies were based on hybridoma technology. However, these attempts were hindered by the lack of an adequate fusion partner for rabbit spleen cells.
Early hybrid cell lines consisting of mouse-rabbit heterohybridomas were extensively studied. But they were found to be unstable and difficult to clone. The first real breakthrough happened in 1995, in a study led by Katherine Knight from Loyola University of Chicago (USA). In this study, scientists were able to generate a suitable fusion partner for rabbit spleen cells from a double transgenic rabbit overexpressing v-abl and c-myc oncogenes. The transgenic rabbit developed a myeloma-like tumor which allowed the isolation of the 240E-1 cell line.
Researchers subsequently merged the 240E-1 cell line with rabbit spleen cells, but the resulting hybridomas were genetically unstable and prone to quickly losing the antibody-encoding genes. The problems of stability were subsequently solved by Weimin Zhu and Robert Pytela, scientists from the University of California San Francisco (USA). These researchers decided to improve 240E-1 stability by repeatedly subcloning and performing medium optimization.
As a result, 240E-1 progeny showed enhanced fusion efficiency and robust growth. The best performing clone from the progeny was named 240E-W. The fusion of this immortal cell line with rabbit spleen cells yielded a great number of stable hybridomas and found to be able to maintain stable growth without losing viability.
Subsequent studies with the 240E-W cell line led to considerable improvements in terms of stability and fusion efficiency. Subsequent cell lines, 240E-W2 (US Patent 7,429,487) and 240E-W3 were further developed to increase fusion efficiency and improve antibody production by eliminating the expression of endogenous rabbit heavy and light chains.
Phage display technology
The process of selecting antibodies through phage display has been described in the early 1990s. However, the adaptation of the technology to rabbit repertoires was only described in 2000 by Christoph Rader and colleagues from The Scripps Research Institute (USA). In this study, the authors applied the robust technique to identify and humanize binders from a naïve scFv rabbit-derived library against the human A33 antigen.
Due to the difficulties associated with the development of rabbit hybridomas, the phage display technology thus became the dominant technology used to produce rabbit monoclonal antibodies. Currently, although it is also possible to perform phage display with the Fab region of rabbit antibodies, the preferred format for many applications is the scFv rabbit/Fab human format.
Concluding remarks
Like murine antibodies, rabbit monoclonal antibodies often need to undergo humanization before they can be considered suitable for clinical application. Techniques previously applied to the humanization of mouse-derived antibodies have been also successfully applied to the humanization of rabbit antibodies. Whether by rational design, directed evolution, or a combination of these methods, it is currently possible to graft all six rabbit CDRs into a human IgG framework.
Nevertheless, until today, only one rabbit-derived antibody has been approved clinical application (Brolucizumab.) For this reason, these antibodies are still preferably used for research, diagnostics, and analytical applications.
- Rossi, S. et al. Rabbit monoclonal antibodies: a comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal antibodies. Am J Clin Pathol. 2005 Aug;124(2):295-302. doi: 10.1309/NR8HN08GDPVEMU08
- Weber, J. et al. From rabbit antibody repertoires to rabbit monoclonal antibodies. Exp Mol Med. 2017; 49(3): e305. doi: 10.1038/emm.2017.23
- Zhang, Y-F. and Ho, M. Humanization of rabbit monoclonal antibodies via grafting combined Kabat/IMGT/Paratome complementarity-determining regions: Rationale and examples. MAbs. 2017 Apr; 9(3): 419–429. doi: 10.1080/19420862.2017.1289302
- Zhang, Z. et al. Advances in the Isolation of Specific Monoclonal Rabbit Antibodies. Front Immunol. 2017; 8:494. doi: 10.3389/fimmu.2017.00494
- Zhu, W. and Yu, G-L. Rabbit Hybridoma. Therapeutic Monoclonal Antibodies: From Bench to Clinic. 2009. Chapter 6. doi: 10.1002/9780470485408.ch6
