 Antibody production
Antibody production
Polyclonal antibody production processes and development for research and diagnostics
Previously, we have discussed the potential benefits and drawbacks of using polyclonal antibodies for research and diagnostics. We have also written about how to choose the best host species for polyclonal antibody production. Here, we will address the different polyclonal antibody production processes. Plus, we will provide an overview of different polyclonal antibody development techniques including purification, fragmentation, and conjugation.
Principles of polyclonal antibody production processes
Polyclonal antibodies are a mixture of distinct monoclonal antibodies generated by different lines of antibody-secreting plasma cells. This mixture of immunoglobulins targets different epitopes (i.e. parts) of a specific antigen. And they can be obtained from different host species including rabbit, chicken, mouse, rat, goat, sheep, or camelids such as llama or alpaca.
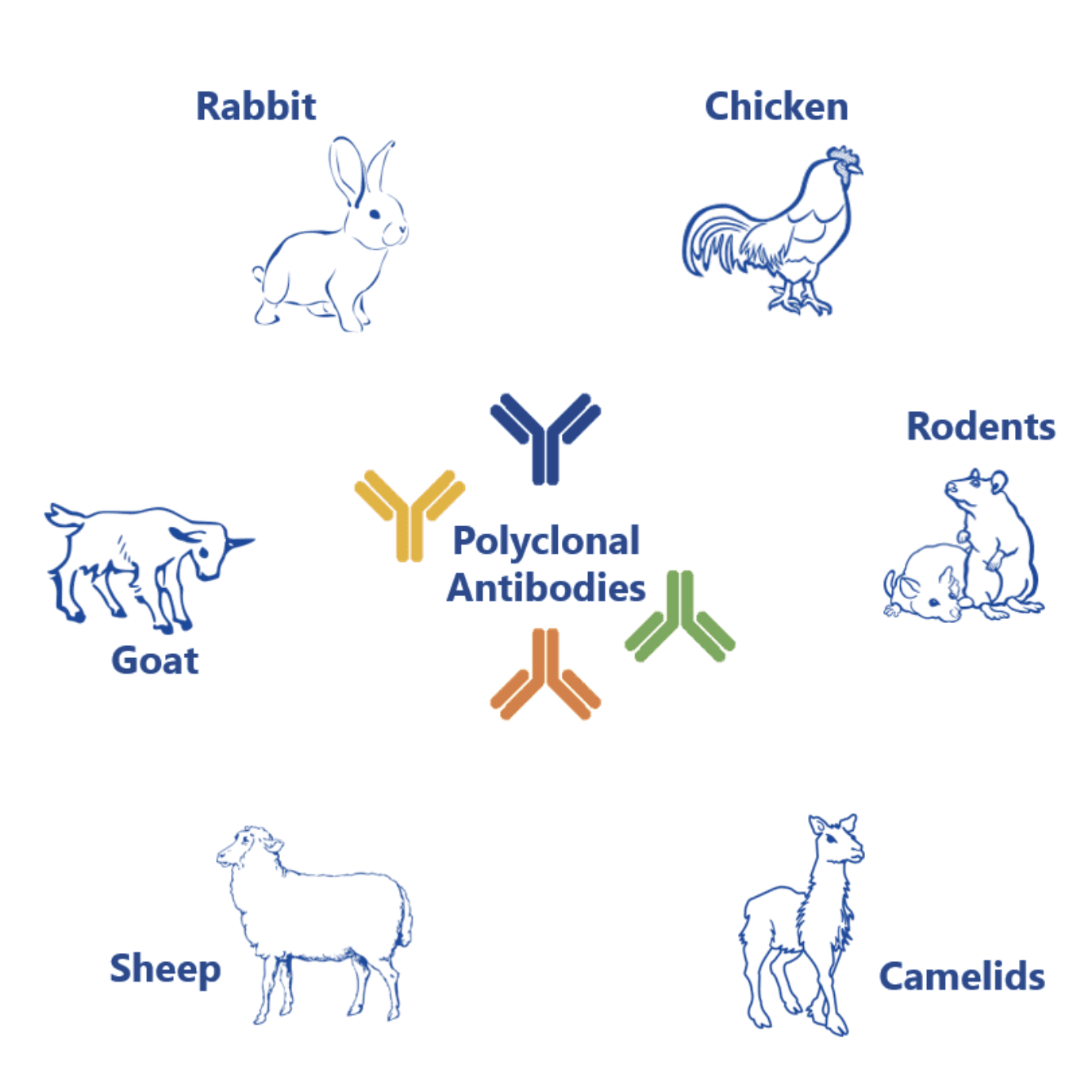
They are more sensitive and robust than monoclonal antibodies. Yet they are also less specific and, thus, have very limited therapeutic value.
Despite these drawbacks, polyclonal antibodies are still invaluable tools for research and diagnostics. The main reason for this is their ability to provide signal amplification. These antibodies can bind to several epitopes of the same antigen. Thus, several antibodies can bind to the same target antigen, leading to a strong signal or more effective capture of the target antigen.
Choosing a species for production is the first step common to all polyclonal antibody production processes. The choice must be taken considering three key aspects:
- Required quantity of antibodies – the size of the host is directly proportional to the final yield. Thus, larger animals should be the preferred hosts when large amounts are required.
- Source of the antigen – the host from which the antigen originated and the host used for antibody production should phylogenetically distant to increase the chances of the antigen eliciting an immune response.
- Final application – polyclonal antibodies used as secondary antibodies (antibodies targeting other antibodies) should be generated in different and phylogenetically distant species than the ones used for primary antibody generation.
Polyclonal antibody production processes are simple and fast compared to monoclonal antibody production. They are generated in vivo by immunizing the chosen host with a specific antigen or hapten–carrier conjugate. Moreover, an adjuvant may be used to enhance the immune response.
Two or three weeks after the first injection, antibody titers are determined. Depending on the required antibody yields, additional injections of the antigen (called “booster”) may be carried out to increase antibody titers. This routine may be repeated several times until the desired antibody titers are achieved.
At this point, polyclonal antibodies are harvested by collecting the blood of the host. The blood is then processed by separating the cellular from the serum fraction. The latter, also called antiserum, contains the antibodies of interest. Additionally, a salt precipitation step can be used to remove unwanted proteins.
Polyclonal antibodies purification processes
Polyclonal antibodies in the crude antiserum may be directly used as detection probes (immunodetection) or purification ligands (immunotechnology). But only a small fraction of this crude mixture (around 10%) has a high affinity against the target antigen.
Hence, antiserum is usually further purified by one or a combination of two processes:
- Immunoglobulin-specific purification
- Antigen-specific purification
They can increase the purity and specificity of polyclonal antibodies. But they also reduce the yields of the final product.
Immunoglobulin-specific purification
The first of these processes relies on the use of immunoglobulin-binding proteins. Bacteria produce these proteins to evade the host immune system. Presently, they have been repurposed to capture soluble immunoglobulins from complex mixtures at an industrial scale.
Currently, three main immunoglobulin-binding proteins are in use. These proteins bind to different regions of the antibody molecule and also have different affinities towards antibodies from different classes and species:
- Protein A, from Staphylococcus aureus, that interacts with the Fc region
- Protein G, from Streptococcus spp., that also interacts with the Fc region
- Protein A/G, a fusion of proteins A and G that combines the binding ability of the two proteins
- Protein L, from Peptostroptococcus magnus, that interacts with the kappa light chain from the Fab region
These proteins may be immobilized on a solid support to capture immunoglobulins from the antiserum. But because they target only conserved regions, they cannot be used to increase the specificity of the final product.
Antigen-specific purification
To collect high-affinity polyclonal antibodies, the antiserum must be purified using antigen-affinity purification. These techniques increase the specificity of the resulting mixture of antibodies and thus reduce the risk of cross-reactivity towards unspecific antigens.
Commonly, to achieve a higher specificity, antibodies can be purified against the target antigen. But many antigens may share similar epitopes. For this reason, the mixture may be further purified using negative selection affinity columns. With this purpose, one or several unspecific antigens can be used to remove antibodies binding to common epitopes and recover the ones that bind to more specific epitopes.
Polyclonal antibody development by fragmentation or conjugation
Purified polyclonal antibodies can be further developed by fragmentation. These molecules can be fragmented into their corresponding functional regions (Fab or Fc) tobroaden their usage for many applications.
Fragmentation is achieved through the use of specific proteases:
- Papain, a cysteine protease present in papaya, that generates monovalent Fab or Fc fragments
- Pepsin, an endopeptidase, that generates bivalent F(ab′)2 fragments.
Papain can cleave antibodies from all IgG subclasses from different hosts. While, pepsin has a narrow substrate range and, thus, its usage is limited to antibodies from specific classes. Hence, alternative methods to generate bivalent F(ab′)2 fragments are currently in use. These methods include the use of papain pre-activated with cysteine or the use of ficain, a protease obtained from figs’ latex.
Polyclonal antibodies or functional fragments thereof can be further conjugated to increase their usefulness. Several reagents are currently in use:
- Biotin can be used to capture specific antigens due to its strong interaction with streptavidin
- Horseradish peroxidase (HRP) can be used as label in Western Blot, ELISA and immunohistochemistry assays
- Fluorescent dyes can be used for direct visualization of target antigens in immunohistochemistry
These methods are especially useful to generate antibodies for a multitude of applications. For instance, Fab and F(ab′)2 fragments can penetrate tissues that are usually inaccessible to full-length antibodies. For this reason, they enhance the efficiency and sensitivity of immunohistochemistry and immunofluorescence techniques.
Moreover, Fc fragments can be used as antigens to generate secondary antibodies targeting the Fc region instead of the whole molecule. In these cases, if the primary antibody is generated in rabbits, polyclonal antibodies from this host can be fragmented and its Fc region used to immunize a host from a different species (e.g. goat, sheep, chicken). This immunization generates antibodies targeting the Fc region of rabbit antibodies, also called anti-rabbit polyclonal antibodies.
These anti-rabbit antibodies can be further fragmented and its Fab regions can be labeled and further paired with a primary rabbit antibody for the indirect detection of a specific antigen.
Concluding remarks
Despite their limited therapeutic application, polyclonal antibodies are invaluable for research and diagnostics.
They provide stronger signals in comparison to monoclonal and recombinant antibodies. Thus, they are useful for the detection of low-abundance or hard-to-reach targets.
Their usefulness can be broadened by the use of different antibody development techniques including purification, fragmentation, and conjugation.
- Akerström, B. et al. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985; 135(4):2589-92. Available from: https://www.ncbi.nlm.nih.gov/pubmed/4031496
- Andrews, S. M. and Titus, J. A. Fragmentation of Immunoglobulin G. Current Protocols in Immunology. 1997; 21(1):2.8.1-2.8.10. doi: 10.1002/0471142735.im0208s21
- Eliasson, M. et al. Chimeric IgG-binding receptors engineered from staphylococcal protein A and streptococcal protein G. J Biol Chem. 1988; 263(9):4323-7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/2964447
- Graille, M. et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: Structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A. 2000; 97(10): 5399–5404. doi: 10.1073/pnas.97.10.5399
- Leenaars, M. and Hendriksen, C. F. M. Critical Steps in the Production of Polyclonal and Monoclonal Antibodies: Evaluation and Recommendations. ILAR Journal. 2005; 46(3):269–279. doi:10.1093/ilar.46.3.269
- Nilson, B. H. et al. Protein L from Peptostreptococcus magnus binds to the kappa light chain variable domain. J Biol Chem. 1992; 267(4):2234-9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/1733930
